Carboxysome
Carboxysomes are bacterial microcompartments (BMCs) consisting of polyhedral protein shells filled with the enzymes ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO)—the predominant enzyme in carbon fixation and the rate limiting enzyme in the Calvin cycle—and carbonic anhydrase.[2]
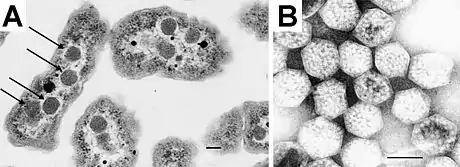
Carboxysomes are thought to have evolved as a consequence of the increase in oxygen concentration in the ancient atmosphere; this is because oxygen is a competing substrate to carbon dioxide in the RuBisCO reaction.[3] To overcome the inefficiency of RuBisCO, carboxysomes concentrate carbon dioxide inside the shell by means of co-localized carbonic anhydrase activity, which produces carbon dioxide from the bicarbonate that diffuses into the carboxysome. The resulting concentration of carbon dioxide near RuBisCO decreases the proportion of ribulose-1,5-bisphosphate oxygenation and thereby avoids costly photorespiratory reactions. The surrounding shell provides a barrier to carbon dioxide loss, helping to increase its concentration around RuBisCO.[4][5] Carboxysomes are an essential part of the carbon dioxide-concentrating mechanism (CCM).
Carboxysomes are the best studied example of bacterial microcompartments, the term for functionally diverse organelles that are alike in having a protein shell.[6][7]
Discovery
Polyhedral bodies were discovered by transmission electron microscopy in the cyanobacterium Phormidium uncinatum in 1956.[8] These were later observed in other cyanobacteria[9] and in some chemotrophic bacteria that fixed carbon dioxide—many of them are sulfur reducers or nitrogen fixers (for example, Halothiobacillus, Acidithiobacillus, Nitrobacter and Nitrococcus; all belonging to Proteobacteria).[2][10] The polyhedral bodies were first purified from Thiobacillus neapolitanus (now Halothiobacillus neapolitanus) in 1973 and shown to contain RuBisCO, held within a rigid outer covering.[11] The authors proposed that since these appeared to be organelles involved in carbon fixation, they should be called carboxysomes.[11]
Architecture

Structurally, carboxysomes are icosahedral, or quasi-icosahedral. Electron cryo-tomography studies[12][13][14] have confirmed the approximately icosahedral geometry of the carboxysome, and have imaged protein molecules inside (presumed to be RuBisCO), arranged in a few concentric layers.[12][14] The non-icosahedral faceted shapes of some carboxysomes can naturally be explained within the elastic theory of heterogeneous thin shells.[15] The carboxysome has an outer shell composed of a few thousand protein subunits, which encapsulates a CO2-producing enzyme (carbonic anhydrase) and a carbon-fixing enzyme (RuBisCO). Proteins known to form the shell have been structurally characterized by X-ray crystallography. The protein that constitutes the majority of the shell forms a cyclical hexamer and belongs to BMC protein family.[16] These hexamers, BMC-H proteins, are the basic building blocks of the shell. In some crystal forms the hexamers assemble further in a side-by-side fashion to form a tightly packed molecular layer, which presumably is how the facets of the shell are assembled. Small pores perforate many different types of BMC-H hexamers, and may serve as the route for diffusion of small substrates (e.g. bicarbonate) into and out of the carboxysome. Positively charged amino acids in the pores presumably help promote the diffusion of the negatively charged substrates and products.[16] Other minor structural components of the shell that have been characterized include pentameric proteins (BMC-P proteins), which have been proposed to occupy the vertices of the icosahedral shell.[17] A third building block of the carboxysome shell is a protein composed of two BMC domains in tandem (BMC-T proteins). Structurally, many of these are known to form trimers which are pseudohexameric.[18][19] Some members of the BMC-T protein family stack in a face-to-face fashion and form tiny cages. Based on crystal structures, these protein cages have relatively large gated pores on both sides, and it has been proposed that the opening and closing of the pore could be controlled in a manner similar to an air-lock. Such an air-lock, in contrast to BMC-H proteins with constitutively open pores, has been suggested to serve as a route for larger substrates (ribulose-1,5-bisphosphate) and products (3-phosphoglycerate) that must cross the shell.[18][19]
A number of viral capsids are also icosahedral, composed of hexameric and pentameric proteins, but currently there is no evidence suggesting any evolutionary relationship between the carboxysome shell and viral capsids.
Two Types of Carboxysomes
There are two types of carboxysomes. Although they may seem similar in appearance, they differ in their protein composition, including the form of RuBisCO they enclose.[20][21] Furthermore, studies have revealed fundamental differences in their gene organization and possibly in how they assemble.
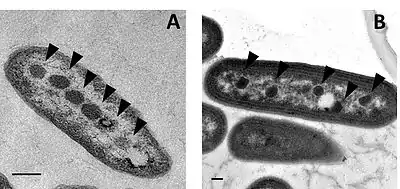
Alpha-Carboxysomes
Alpha-carboxysomes (aka α-carboxysomes) are also referred as the cso type of carboxysome. They contain Form IA RuBisCO; they are found in alpha-cyanobacteria, some nitrifying bacteria, some sulfur-oxidizing bacteria (for example, Halothiobacillus neapolitanus), and some purple bacteria; these are all classified as Proteobacteria). The alpha-carboxysome was the first bacterial microcompartment to be purified and characterized.[22][23] Electron microscopy studies on purified alpha-carboxysomes or cell sections containing alpha-carboxysomes revealed that they are typically 100-160 nm in diameter.[24] Common building blocks for the shell of alpha-carboxysomes are called CsoS1A/B/C (BMC-H), CsoS4A/B (BMC-P), and CsoS1D (BMC-T). CsoS4A/B were the first BMC-P proteins to be experimentally demonstrated as minor components of the BMC shell[4] (only 12 pentamers are required to cap the vertices of an icosahedron). CsoS1D is first BMC-T which has been structurally characterized; it is also the first example of dimerization of two BMC building blocks in a face-to-face fashion to create a tiny cage. The CsoS1D cage has gated pore at both end, which is proposed to facilitate large metabolites crossing the shell.[19] In addition to the specific form of RuBisCO, other encapsulated proteins distinguish alpha-carboxysomes from beta-carboxysomes such as CsoS2 and CsoSCA. The CsoS2 protein has a very high pI and a unique primary structure. The primary structure of CsoS2 appears tripartite, composed of an N-terminal, middle- and C-terminal regions.[25] Repetitive motifs can be identified in the N-terminal and middle regions. Recently, it was proposed to be an intrinsically disordered protein with an essential role in alpha-carboxysome assembly. CsoSCA is a shell-associated beta-carbonic anhydrase.[5][26] Studies in Halothiobacillus neapolitanus have shown that empty shells of normal shape and composition are assembled in carboxysomal RuBisCO-lacking mutants, suggesting that alpha-carboxysome shell biogenesis and enzyme sequestration are two independent, but functionally linked processes.[27] Intriguingly, carboxysomes of Halothiobacillus neapolitanus have been found to accommodate chimeric and heterologous species of RuBisCO and it is the large subunit of RuBisCO which determines whether the enzyme is sequestered into carboxysomes or not.[27]
Beta-carboxysomes
Beta-carboxysomes (aka β-carboxysomes) can be found in cyanobacteria.[28]
The signature proteins of the beta-carboxysome are Form IB RuBisCO and a gamma carbonic anhydrase homolog.[6] Beta-carboxysomes are typically bigger than alpha-carboxysomes: the observed diameters for them vary from 200 to 400 nm.[25] The structural proteins that are essential for carboxysome formation are encoded in the conserved carboxysome locus[7] known as the ccm locus. The ccm locus includes genes for core proteins CcmM and CcmN and the shell proteins CcmK (a BMC-H protein), CcmL (a BMC-P protein) and CcmO (a BMC-T protein).
A full length CcmM protein consists of a gamma-carbonic anhydrase domain on and three to five small subunit-like domains (SSLDs; which resemble RbcS, the small subunit of RuBisCO) on its C-terminus.[29] The ccmM gene contains an internal translation site that produces a short form of CcmM (a protein which only consists of SSLDs); both long and short forms of CcmM are required for carboxysome assembly.[30] CcmN contains multiple hexapeptide-repeat domains on its N-terminus and a short α-helical encapsulation peptide on the C-terminus.[31]
Other structural components of the carboxysomes are encoded outside of the ccm locus. CcmP is a BMC-T protein that is absolutely conserved among organisms that form beta-carboxysomes. CcmP pseudohexamer stacks to form a nanocompartment—an example of an air-lock forming protein.[18] Likewise, in some cyanobacterial strains the beta-carboxysomes contain a beta-carbonic anhydrase that is not found in the ccm locus.[32]
The beta-carboxysome assembles from the inside out, first an enzymatic core forms that is subsequently encapsulated by a protein shell.[33] Carboxysome assembly occurs through a series of protein-protein interactions: the enzyme RuBisCO and the two isoforms (full length and short form) of the CcmM protein interact by means of the SSLDs; in strains containing CcaA the beta-carbonic anhydrase is brought into the carboxysome core by interaction with the N-terminus of the full length CcmM.[34][35] Once the procarboxysome (the carboxysome core) is formed, the N-terminus of the adapter protein CcmN interacts with the N-terminus of CcmM, while the C-terminus of CcmN recruits the shell proteins CcmK (BMC-H) and CcmO (BMC-T).[31] The final step is the addition of the vertices formed by the BMC-P protein CcmL, which then fully cap the enzymatic core.[33]
Potential uses of the carboxysome in biotechnology
As the case with other BMCs, the carboxysome is attracting significant attention by researchers for applications in synthetic biology. The transfer of a genetic module coding for an alpha-carboxysome has been shown to produce carboxysome-like structures in E. coli.[36] Bioengineering of carboxysome shells have been shown feasible and beta-carboxysomes constructed with chimeric proteins or with chimeric shells has been reported.[37] The introduction of carboxysomes into plant chloroplasts as part of a CO2 concentrating mechanism [38] [39] (such as that found in cyanobacteria) is predicted to have significant improvements on net CO2 fixation and yield.[40][41] Expression of beta-carboxysomal shell proteins [42] and Form IB Rubisco-CcmM complexes in tobacco chloroplasts has been achieved,[43] but this did not result in compartments containing Rubisco. A further advance has been the construction of minimal alpha-carboxysomes from the cyanobacterium Cyanobium PCC7001 in tobacco chloroplasts [44] containing Form IA Rubisco and the CsoS1A and CsoS2 proteins. As yet, identifiably functional carboxysomes have not yet been constructed in plant chloroplasts. Nonetheless, successful improvement of photosynthesis in plants using this approach is ultimately dependent on the operation of transporter proteins in the chloroplast inner envelope membrane to help generate a high concentration of bicarbonate inside the chloroplast.[45]
References
- Tsai Y, Sawaya MR, Cannon GC, et al. (June 2007). "Structural Analysis of CsoS1A and the Protein Shell of the Halothiobacillus neapolitanus Carboxysome". PLOS Biol. 5 (6): e144. doi:10.1371/journal.pbio.0050144. PMC 1872035. PMID 17518518.
- Yeates, Todd O.; Kerfeld, Cheryl A.; Heinhorst, Sabine; Cannon, Gordon C.; Shively, Jessup M. (2008). "Protein-based organelles in bacteria: carboxysomes and related microcompartments". Nature Reviews Microbiology. 6 (9): 681–691. doi:10.1038/nrmicro1913. ISSN 1740-1526. PMID 18679172.
- Badger, M. R. (2003). "CO2 concentrating mechanisms in cyanobacteria: molecular components, their diversity and evolution". Journal of Experimental Botany. 54 (383): 609–622. doi:10.1093/jxb/erg076. ISSN 1460-2431. PMID 12554704.
- Cai, Fei; Menon, Balaraj B.; Cannon, Gordon C.; Curry, Kenneth J.; Shively, Jessup M.; Heinhorst, Sabine (2009). "The Pentameric Vertex Proteins Are Necessary for the Icosahedral Carboxysome Shell to Function as a CO2 Leakage Barrier". PLOS ONE. 4 (10): e7521. doi:10.1371/journal.pone.0007521. ISSN 1932-6203. PMC 2760150. PMID 19844578.
- Dou, Z.; Heinhorst, S.; Williams, E. B.; Murin, C. D.; Shively, J. M.; Cannon, G. C. (2008). "CO2 Fixation Kinetics of Halothiobacillus neapolitanus Mutant Carboxysomes Lacking Carbonic Anhydrase Suggest the Shell Acts as a Diffusional Barrier for CO2". Journal of Biological Chemistry. 283 (16): 10377–10384. doi:10.1074/jbc.M709285200. ISSN 0021-9258. PMID 18258595.
- Kerfeld, Cheryl A.; Erbilgin, Onur (2015). "Bacterial microcompartments and the modular construction of microbial metabolism". Trends in Microbiology. 23 (1): 22–34. doi:10.1016/j.tim.2014.10.003. ISSN 0966-842X. PMID 25455419.
- Axen, Seth D.; Erbilgin, Onur; Kerfeld, Cheryl A. (2014). "A Taxonomy of Bacterial Microcompartment Loci Constructed by a Novel Scoring Method". PLOS Computational Biology. 10 (10): e1003898. doi:10.1371/journal.pcbi.1003898. ISSN 1553-7358. PMC 4207490. PMID 25340524.
- G. DREWS & W. NIKLOWITZ (1956). "[Cytology of Cyanophycea. II. Centroplasm and granular inclusions of Phormidium uncinatum]". Archiv für Mikrobiologie. 24 (2): 147–162. PMID 13327992.
- E. Gantt & S. F. Conti (March 1969). "Ultrastructure of blue-green algae". Journal of Bacteriology. 97 (3): 1486–1493. doi:10.1128/JB.97.3.1486-1493.1969. PMC 249872. PMID 5776533.
- Shively, J M (1974). "Inclusion Bodies of Prokaryotes". Annual Review of Microbiology. 28 (1): 167–188. doi:10.1146/annurev.mi.28.100174.001123. ISSN 0066-4227. PMID 4372937.
- Shively, J. M.; Ball, F.; Brown, D. H.; Saunders, R. E. (1973). "Functional Organelles in Prokaryotes: Polyhedral Inclusions (Carboxysomes) of Thiobacillus neapolitanus". Science. 182 (4112): 584–586. doi:10.1126/science.182.4112.584. ISSN 0036-8075. PMID 4355679.
- Iancu, Cristina V.; Ding, H. Jane; Morris, Dylan M.; Dias, D. Prabha; Gonzales, Arlene D.; Martino, Anthony; Jensen, Grant J. (2007). "The Structure of Isolated Synechococcus Strain WH8102 Carboxysomes as Revealed by Electron Cryotomography". Journal of Molecular Biology. 372 (3): 764–773. doi:10.1016/j.jmb.2007.06.059. ISSN 0022-2836. PMC 2453779. PMID 17669419.
- Iancu, Cristina V.; Morris, Dylan M.; Dou, Zhicheng; Heinhorst, Sabine; Cannon, Gordon C.; Jensen, Grant J. (2010). "Organization, Structure, and Assembly of α-Carboxysomes Determined by Electron Cryotomography of Intact Cells". Journal of Molecular Biology. 396 (1): 105–117. doi:10.1016/j.jmb.2009.11.019. ISSN 0022-2836. PMC 2853366. PMID 19925807.
- Schmid, Michael F.; Paredes, Angel M.; Khant, Htet A.; Soyer, Ferda; Aldrich, Henry C.; Chiu, Wah; Shively, Jessup M. (2006). "Structure of Halothiobacillus neapolitanus Carboxysomes by Cryo-electron Tomography". Journal of Molecular Biology. 364 (3): 526–535. doi:10.1016/j.jmb.2006.09.024. hdl:11147/2128. ISSN 0022-2836. PMC 1839851. PMID 17028023.
- Vernizzi, G.; Sknepnek, R.; Olvera de la Cruz, M. (2011). "Platonic and Archimedean geometries in multicomponent elastic membranes". Proceedings of the National Academy of Sciences. 108 (11): 4292–4296. doi:10.1073/pnas.1012872108. ISSN 0027-8424. PMC 3060260. PMID 21368184.
- Kerfeld, C. A. (2005). "Protein Structures Forming the Shell of Primitive Bacterial Organelles". Science. 309 (5736): 936–938. CiteSeerX 10.1.1.1026.896. doi:10.1126/science.1113397. ISSN 0036-8075. PMID 16081736.
- Tanaka, S.; Kerfeld, C. A.; Sawaya, M. R.; Cai, F.; Heinhorst, S.; Cannon, G. C.; Yeates, T. O. (2008). "Atomic-Level Models of the Bacterial Carboxysome Shell". Science. 319 (5866): 1083–1086. doi:10.1126/science.1151458. ISSN 0036-8075. PMID 18292340. S2CID 5734731.
- Cai, F.; Sutter, M.; Cameron, J. C.; Stanley, D. N.; Kinney, J. N.; Kerfeld, C. A. (2013). "The Structure of CcmP, a Tandem Bacterial Microcompartment Domain Protein from the ?-Carboxysome, Forms a Subcompartment Within a Microcompartment". Journal of Biological Chemistry. 288 (22): 16055–16063. doi:10.1074/jbc.M113.456897. ISSN 0021-9258. PMC 3668761. PMID 23572529.
- Klein, Michael G.; Zwart, Peter; Bagby, Sarah C.; Cai, Fei; Chisholm, Sallie W.; Heinhorst, Sabine; Cannon, Gordon C.; Kerfeld, Cheryl A. (2009). "Identification and Structural Analysis of a Novel Carboxysome Shell Protein with Implications for Metabolite Transport" (PDF). Journal of Molecular Biology. 392 (2): 319–333. doi:10.1016/j.jmb.2009.03.056. hdl:1721.1/61355. ISSN 0022-2836. PMID 19328811.
- Zarzycki, J.; Axen, S. D.; Kinney, J. N.; Kerfeld, C. A. (2012). "Cyanobacterial-based approaches to improving photosynthesis in plants". Journal of Experimental Botany. 64 (3): 787–798. doi:10.1093/jxb/ers294. ISSN 0022-0957. PMID 23095996.
- Rae, B. D.; Long, B. M.; Badger, M. R.; Price, G. D. (2013). "Functions, Compositions, and Evolution of the Two Types of Carboxysomes: Polyhedral Microcompartments That Facilitate CO2 Fixation in Cyanobacteria and Some Proteobacteria". Microbiology and Molecular Biology Reviews. 77 (3): 357–379. doi:10.1128/MMBR.00061-12. ISSN 1092-2172. PMC 3811607. PMID 24006469.
- Shively JM, Bock E, Westphal K, Cannon GC (November 1977). "Icosahedral inclusions (carboxysomes) of Nitrobacter agilis". Journal of Bacteriology. 132 (2): 673–675. doi:10.1128/JB.132.2.673-675.1977. PMC 221910. PMID 199579.
- Cannon, G. C.; Shively, J. M. (1983). "Characterization of a homogenous preparation of carboxysomes from Thiobacillus neapolitanus". Archives of Microbiology. 134 (1): 52–59. doi:10.1007/BF00429407. ISSN 0302-8933.
- Heinhorst, Sabine; Cannon, Gordon C.; Shively, Jessup M. (2014). Carboxysomes and Their Structural Organization in Prokaryotes. Nanomicrobiology. pp. 75–101. doi:10.1007/978-1-4939-1667-2_4. ISBN 978-1-4939-1666-5.
- Cai, Fei; Dou, Zhicheng; Bernstein, Susan; Leverenz, Ryan; Williams, Eric; Heinhorst, Sabine; Shively, Jessup; Cannon, Gordon; Kerfeld, Cheryl (2015). "Advances in Understanding Carboxysome Assembly in Prochlorococcus and Synechococcus Implicate CsoS2 as a Critical Component". Life. 5 (2): 1141–1171. doi:10.3390/life5021141. ISSN 2075-1729. PMC 4499774. PMID 25826651.
- Sawaya, M. R.; Cannon, G. C.; Heinhorst, S.; Tanaka, S.; Williams, E. B.; Yeates, T. O.; Kerfeld, C. A. (2006). "The Structure of beta-Carbonic Anhydrase from the Carboxysomal Shell Reveals a Distinct Subclass with One Active Site for the Price of Two". Journal of Biological Chemistry. 281 (11): 7546–7555. doi:10.1074/jbc.M510464200. ISSN 0021-9258. PMID 16407248.
- Menon, Balaraj B.; Dou, Zhicheng; Heinhorst, Sabine; Shively, Jessup M.; Cannon, Gordon C. (2008). "Halothiobacillus neapolitanus Carboxysomes Sequester Heterologous and Chimeric RubisCO Species". PLOS ONE. 3 (10): e3570. doi:10.1371/journal.pone.0003570. ISSN 1932-6203. PMC 2570492. PMID 18974784.
- Manuel Sommer, Fei Cai, Matthew Melnicki, Cheryl A Kerfeld: β-Carboxysome bioinformatics: identification and evolution of new bacterial microcompartment protein gene classes and core locus constraints. In: J Exp Bot. 68(14). 2017 Jun 22. Pp 3841–3855. Published online 2017 Apr 17. doi:10.1093/jxb/erx115. PMC 5853843. PMID 28419380
- Long, B. M.; Badger, M. R.; Whitney, S. M.; Price, G. D. (2007). "Analysis of Carboxysomes from Synechococcus PCC7942 Reveals Multiple Rubisco Complexes with Carboxysomal Proteins CcmM and CcaA". Journal of Biological Chemistry. 282 (40): 29323–29335. doi:10.1074/jbc.M703896200. ISSN 0021-9258. PMID 17675289.
- Long, B. M.; Tucker, L.; Badger, M. R.; Price, G. D. (2010). "Functional Cyanobacterial ?-Carboxysomes Have an Absolute Requirement for Both Long and Short Forms of the CcmM Protein". Plant Physiology. 153 (1): 285–293. doi:10.1104/pp.110.154948. ISSN 0032-0889. PMC 2862411. PMID 20304968.
- Kinney, J. N.; Salmeen, A.; Cai, F.; Kerfeld, C. A. (2012). "Elucidating Essential Role of Conserved Carboxysomal Protein CcmN Reveals Common Feature of Bacterial Microcompartment Assembly". Journal of Biological Chemistry. 287 (21): 17729–17736. doi:10.1074/jbc.M112.355305. ISSN 0021-9258. PMC 3366800. PMID 22461622.
- Cannon, Gordon C.; Heinhorst, Sabine; Kerfeld, Cheryl A. (2010). "Carboxysomal carbonic anhydrases: Structure and role in microbial CO2 fixation". Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics (Submitted manuscript). 1804 (2): 382–392. doi:10.1016/j.bbapap.2009.09.026. ISSN 1570-9639. PMID 19818881.
- Cameron, Jeffrey?C.; Wilson, Steven?C.; Bernstein, Susan?L.; Kerfeld, Cheryl?A. (2013). "Biogenesis of a Bacterial Organelle: The Carboxysome Assembly Pathway". Cell. 155 (5): 1131–1140. doi:10.1016/j.cell.2013.10.044. ISSN 0092-8674. PMID 24267892.
- Cot, S. S.-W.; So, A. K.-C.; Espie, G. S. (2007). "A Multiprotein Bicarbonate Dehydration Complex Essential to Carboxysome Function in Cyanobacteria". Journal of Bacteriology. 190 (3): 936–945. doi:10.1128/JB.01283-07. ISSN 0021-9193. PMC 2223583. PMID 17993516.
- Long, Benedict M.; Rae, Benjamin D.; Badger, Murray R.; Dean Price, G. (2011). "Over-expression of the β-carboxysomal CcmM protein in Synechococcus PCC7942 reveals a tight co-regulation of carboxysomal carbonic anhydrase (CcaA) and M58 content". Photosynthesis Research. 109 (1–3): 33–45. doi:10.1007/s11120-011-9659-8. ISSN 0166-8595. PMID 21597987.
- Bonacci, W.; Teng, P. K.; Afonso, B.; Niederholtmeyer, H.; Grob, P.; Silver, P. A.; Savage, D. F. (2011). "Modularity of a carbon-fixing protein organelle". Proceedings of the National Academy of Sciences. 109 (2): 478–483. doi:10.1073/pnas.1108557109. ISSN 0027-8424. PMC 3258634. PMID 22184212.
- Cai, Fei; Sutter, Markus; Bernstein, Susan L.; Kinney, James N.; Kerfeld, Cheryl A. (2015). "Engineering Bacterial Microcompartment Shells: Chimeric Shell Proteins and Chimeric Carboxysome Shells". ACS Synthetic Biology. 4 (4): 444–453. doi:10.1021/sb500226j. ISSN 2161-5063. PMID 25117559.
- Price, GD; Badger, MR (2008). "Advances in understanding the cyanobacterial CO2-concentrating-mechanism (CCM): Functional components, Ci transporters, diversity, genetic regulation and prospects for engineering into plants". Journal of Experimental Botany. 59 (7): 1441–1461. doi:10.1093/jxb/erm112. PMID 17578868.
- Price, GD; Pengelly, JJ (2013). "The cyanobacterial CCM as a source of genes for improving photosynthetic CO2 fixation in crop species". Journal of Experimental Botany. 64 (3): 753–768. doi:10.1093/jxb/ers257. PMID 23028015.
- McGrath, JM; Long, SP (2014). "Can the cyanobacterial carbon-concentrating mechanism increase photosynthesis in crop species? A theoretical analysis". Plant Physiology. 164 (4): 2247–61. doi:10.1104/pp.113.232611. PMC 3982776. PMID 24550242.
- Yin, X; Struik, PC (2017). "Can increased leaf photosynthesis be converted into higher crop mass production? A simulation study for rice using the crop model GECROS". Journal of Experimental Botany. 68 (9): 2345–2360. doi:10.1093/jxb/erx085. PMC 5447886. PMID 28379522.
- Lin, Myat T.; Occhialini, Alessandro; Andralojc, P. John; Devonshire, Jean; Hines, Kevin M.; Parry, Martin A. J.; Hanson, Maureen R. (2014). "α-Carboxysomal proteins assemble into highly organized structures in Nicotianachloroplasts". The Plant Journal. 79 (1): 1–12. doi:10.1111/tpj.12536. ISSN 0960-7412. PMC 4080790. PMID 24810513.
- Lin, Myat T.; Occhialini, Alessandro; Andralojc, P. John; Parry, Martin A. J.; Hanson, Maureen R. (2014). "A faster Rubisco with potential to increase photosynthesis in crops". Nature. 513 (7519): 547–550. doi:10.1038/nature13776. ISSN 0028-0836. PMC 4176977. PMID 25231869.
- Long, BM; Hee, WY (2018). "Carboxysome encapsulation of the CO2-fixing enzyme Rubisco in tobacco chloroplasts". Nature Communications. 9 (1): 3570. doi:10.1038/s41467-018-06044-0. PMC 6120970. PMID 30177711.
- Rae, BD; Long, BM (2017). "Progress and challenges of engineering a biophysical carbon dioxide-concentrating mechanism into higher plants". Journal of Experimental Botany. 68 (14): 717–3737. doi:10.1093/jxb/erx133. PMID 28444330..