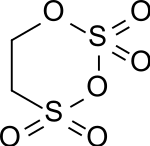
Carbyl sulfate
Carbyl sulfate is an organosulfur compound. The white solid is the product of the reaction of sulfur trioxide and ethylene. It is used in preparation of some dyes and other organosulfur compounds.[1] Carbyl sulfate is a colorless, crystalline, hygroscopic substance although commercial product can appear as a liquid. Because of its unpleasant properties carbyl sulfate is difficult to handle and is usually not isolated but further processed to give secondary products.
 | |
| Names | |
|---|---|
| IUPAC name
1,3,2,4-Dioxadithiane 2,2,4,4-tetraoxide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.007.244 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C2H4O6S2 | |
| Molar mass | 188.17 g·mol−1 |
| Appearance | White solid |
| Melting point | 107.5–109 °C (225.5–228.2 °F; 380.6–382.1 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Production
Regnault[2] and Heinrich Gustav Magnus[3][4] reported first in the years 1838 to 1839 on the compound as reaction product of anhydrous ethanol and anhydrous sulfuric acid.
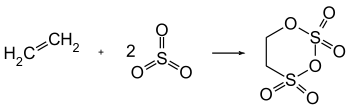
Carbyl sulfate is produced in the highly exothermic (about 800 kcal/kg) reaction of ethylene and sulfur trioxide in the vapor phase in nearly quantitative yield.[5][6]
Disulfuric acid and chlorosulfuric acid can also be used as a sulfonating agent, replacing sulfur trioxide. Instead of ethylene, ethylene-forming agents can be used, e.g. ethanol or diethyl ether.[5]
The product of industrial processes is a water-clear liquid which has - in accordance with D.S. Breslow[5] (107.5 to 109 °C) - a melting range from 102 to 108 °C. Previously stated melting point of about 80 °C[4] results from adhering sulfur trioxide.[5]
Reactions and use
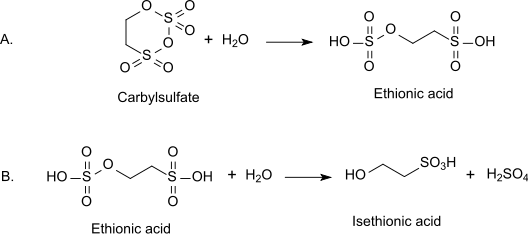
As a cyclic sulfate ester, it is an alkylating agent. Hydrolysis affords ethionic acid, which retains one sulfate ester group. Ethionic acid undergoes further hydrolysis to isethionic acid:
Carbyl sulfate is used as precursor for vinylsulfonic acid and sodium vinyl sulfonate, which are important activated alkenes and are used e. g. as anionic comonomers. A number of functional compounds with a variety of applications are available by nucleophilic addition at the activated double bond of the vinyl sulfonic acid and its derivatives.[7]
Safety
The material is highly reactive. It can decompose explosively when heated above 170 °C.
References
- Kosswig, Kurt (2000). Sulfonic Acids, Aliphatic. Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH. doi:10.1002/14356007.a25_503. ISBN 978-3-527-30673-2.
- Regnault, V. (1838). "Ueber die Einwirkung der wasserfreien Schwefelsäure auf Doppeltkohlenwasserstoff". Annalen der Pharmacie. 25: 32–47. doi:10.1002/jlac.18380250103.
- "Ueber das Carbylsulphat und die Aethionsäure". Annalen der Pharmacie. 32 (3): 249–258. 1839. doi:10.1002/jlac.18390320310.
- "Zur Erinnerung an Gustav Magnus". Nach einem am 14. December 1870 in der General-Versammlung der Deutschen Chemischen Gesellschaft zu Berlin gehaltenen Vortrage August Wilhelm Hofmann s, Berlin, Ferd. Dümmler's Verlagsbuchhandlung, 1871 (S. 32)
- Breslow, David S.; Hough, Robert R. (1957), "The Synthesis of Sodium Ethylenesulfonate from Ethylene", Journal of the American Chemical Society (in German), 79 (18), pp. 5000–5002, doi:10.1021/ja01575a046
- DE 2509738 "Verfahren zur Herstellung von Carbylsulfat." Inventor: Rudolf Irnich, Rolf Schneider
- H. Distler (1965-04-07). "Zur Chemie der Vinylsulfonsäure". Angewandte Chemie. 77 (7): 291–302. doi:10.1002/ange.19650770704.
Further reading
- Breslow, David S.; Hough, Robert R. (1957). "Synthesis of sodium ethylenesulfonate from ethylene". Journal of the American Chemical Society. 79 (18): 5000–5002. doi:10.1021/ja01575a046.