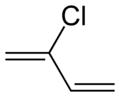
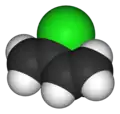
Chloroprene
Chloroprene is the common name for 2-chlorobuta-1,3-diene (IUPAC name) with the chemical formula CH2=CCl−CH=CH2.[3] Chloroprene is a colorless volatile liquid, almost exclusively used as a monomer for the production of the polymer polychloroprene, a type of synthetic rubber. Polychloroprene is better known as Neoprene, the trade name given by DuPont.
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
2-Chlorobuta-1,3-diene | |||
| Other names
Chloroprene, 2-chloro-1,3-butadiene, Chlorobutadiene, β-Chloroprene | |||
| Identifiers | |||
3D model (JSmol) |
|||
| 741875 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.004.381 | ||
| EC Number |
| ||
| 277888 | |||
| KEGG | |||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1991 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C4H5Cl | |||
| Molar mass | 88.5365 g/mol | ||
| Appearance | Colorless liquid | ||
| Odor | pungent, ether-like | ||
| Density | 0.9598 g/cm3 | ||
| Melting point | −130 °C (−202 °F; 143 K) | ||
| Boiling point | 59.4 °C (138.9 °F; 332.5 K) | ||
| 0.026 g/100 mL | |||
| Solubility | soluble in alcohol, diethyl ether miscible in ethyl ether, acetone, benzene | ||
| Vapor pressure | 188 mmHg (20 °C)[1] | ||
Refractive index (nD) |
1.4583 | ||
| Hazards | |||
| Main hazards | Highly flammable, irritant, toxic. | ||
| GHS pictograms |    | ||
| GHS Signal word | Danger | ||
| H225, H302, H315, H319, H332, H335, H350, H373 | |||
| P201, P202, P210, P233, P240, P241, P242, P243, P260, P261, P264, P270, P271, P280, P281, P301+312, P302+352, P303+361+353, P304+312, P304+340, P305+351+338, P308+313, P312, P314, P321 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −15.6 °C (3.9 °F; 257.5 K) | ||
| Explosive limits | 1.9%–11.3%[1] | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
450 mg/kg (rat, oral) | ||
LC50 (median concentration) |
3207 ppm (rat, 4 hr)[2] | ||
LCLo (lowest published) |
1052 ppm (rabbit, 8 hr) 350 ppm (cat, 8 hr)[2] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
TWA 25 ppm (90 mg/m3) [skin][1] | ||
REL (Recommended) |
Ca C 1 ppm (3.6 mg/m3) [15-minute][1] | ||
IDLH (Immediate danger) |
300 ppm[1] | ||
| Related compounds | |||
Related Dienes |
Butadiene Isoprene | ||
Related compounds |
Vinyl chloride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
History
Although it may have been discovered earlier, chloroprene was largely developed by DuPont during the early 1930s, specifically with the formation of neoprene in mind.[4] The chemists Elmer K. Bolton, Wallace Carothers, Arnold Collins and Ira Williams are generally accredited with its development and commercialisation although the work was based upon that of Julius Arthur Nieuwland, with whom they collaborated.[5]
Production
Chloroprene is produced in three steps from 1,3-butadiene: (i) chlorination, (ii) isomerization of part of the product stream, and (iii) dehydrochlorination of 3,4-dichlorobut-1-ene.
Chlorine adds to 1,3-butadiene to afford a mixture of 3,4-dichlorobut-1-ene and 1,4-dichlorobut-2-ene. The 1,4-dichloro isomer is subsequently isomerized to 3,4 isomer, which in turn is treated with base to induce dehydrochlorination to 2-chlorobuta-1,3-diene. This dehydrohalogenation entails loss of a hydrogen atom in the 3 position and the chlorine atom in the 4 position thereby forming a double bond between carbons 3 and 4. In 1983, approximately 2,000,000 kg was produced in this manner.[3] The chief impurity in chloroprene prepared in this way is 1-chlorobuta-1,3-diene, which is usually separated by distillation.
Acetylene process
Until the 1960s, chloroprene production was dominated by the "acetylene process," which was modeled after the original synthesis of vinylacetylene.[4] In this process, acetylene is dimerized to give vinyl acetylene, which is then combined with hydrogen chloride to afford 4-chloro-1,2-butadiene (an allene derivative), which in the presence of copper(I) chloride, rearranges to the targeted 2-chlorobuta-1,3-diene:[3]
This process is energy-intensive and has high investment costs. Furthermore, the intermediate vinyl acetylene is unstable. This "acetylene process" has been replaced by a process, which adds Cl2 to one of the double bonds in 1,3-butadiene, and subsequent elimination produces HCl instead, as well as chloroprene.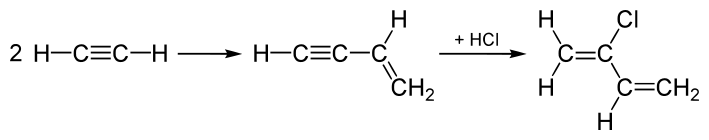
Regulations
Transportation
Only stabilized chloroprene can be transported in U.S..
Occupational health and safety
It is in hazard class 3 (flammable liquid). Its UN number is 1991 and is in packing group 1.
Hazards



.png.webp)

As a way to visually communicate hazards associated with chloroprene exposure, the United Nations Globally Harmonized System of Classification and Labeling of Chemicals has designated the following hazards for exposure to chloroprene: flammable, toxic, dangerous to the environment, health hazard and irritant. Chloroprene poses fire hazard (flash point -4 °F).[8] OSHA identifies chloroprene as a category 2 flammable liquid and emphasizes that at least one portable fire extinguisher should be within 10 and no more than 25 feet away from the flammable liquid storage area.[9] OSHA provides resources on addressing flammable liquids at industrial plants which is where the likely exposure to chloroprene exists (see external resources). As a vapor, chloroprene is heavier than air.
According to the National Fire Protection Association's rating system, chloroprene is designated with a category 2 health hazard (temporary incapacitation or residual injury), a category 3 fire hazard (ignition under the presence of moderate heat), and a category 1 reactivity (unstable at high temperatures and pressures).[10][11]
Chronic exposure to chloroprene may have the following symptoms: liver function abnormalities, disorders of the cardiovascular system, and depression of the immune system.[8]
The Environmental Protection Agency designated chloroprene as likely to be carcinogenic to humans based on evidence from studies that showed a statistically significant association between occupational chloroprene exposure and the risk of lung cancer.[12] As early as 1975, NIOSH had identified the potential health hazards of chloroprene in their bulletin primarily citing two Russian cohort studies from those working with chloroprene in an occupational setting.[13]
Hazard determination
OSHA defines hazard determination as "the process of evaluating available scientific evidence in order to determine if a chemical is hazardous pursuant to the HCS." While chemical manufacturers and importers are required to conduct a hazard determination, other companies may voluntarily conduct a hazard determination to ensure worker health and safety. Under the hazard determination framework, any chemical that has a physical or health hazard is considered a hazardous chemical. Physical hazards include fire hazards, reactive hazards, and explosion hazards. Heath hazards include systemic effects and target organ effects. Chloroprene is on OSHA's list for substances that are regulated as toxic and hazardous.[14]
In the European Union, the hazard-determination-equivalent is the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation enacted on June 1, 2007 by the European Chemicals Agency (ECHA). The goal of REACH is to "improve the protection of human health and the environment from the risks that can be posed by chemicals, while enhancing the competitiveness of the EU chemicals industry."[15] If risks of chemicals are unmanageable, ECHA may ban its use.
Hazard controls
Several epidemiological studies and toxicological reports provide evidence of chloroprene's capability to inflict occupational health and safety concerns. However, varying reviews of the degree to which chloroprene should be held responsible for health concerns highlight the criticality of sound scientific research.[16] Nonetheless, health and safety practices should always be implemented in the workplace. Some of these occupational concerns include: cleaning equipment or unclogging pipes coated with chloroprene, inhaling chloroprene off-gas, chloroprene spontaneously reacting with other chemicals and chloroprene inducing a workplace fire. Upon the clogging of equipment associated with occupational chloroprene use, employers should ensure that their employees are wearing the proper PPE and set up administrative controls so that skin exposure to and inhalation of chloroprene is avoided.[17][18] Only one fatality as a result of chloroprene intoxication has been recorded which was a result of cleaning a container used for chloroprene.[18]
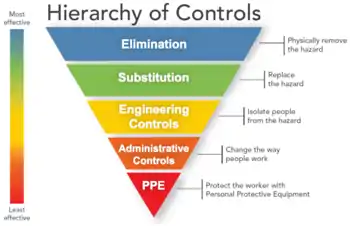
The primary occupational concern for chloroprene is limited to the facilities producing chloroprene and using chloroprene to produce the synthetic rubber, polychloroprene.[12] NIOSH developed a list of actions to address specific workplace hazards. These actions are represented in their diagram of the "Hierarchy of Controls" shown below with the most effective steps at the top and the least effective at the bottom.
The high vaporization potential and flammability of chloroprene has significant implications for handling and storage operations in the occupational setting. Chloroprene should be stored in closed containers in a cool, well-ventilated area with the temperature no higher than 50 degrees Fahrenheit. In addition, chloroprene has a high reactivity and should be stored away from oxidizing agents such as perchlorate, peroxides, permanganates, chlorates, nitrates, chlorine, bromine, and fluorine. All activities inducing a potential fire hazard should be avoided. For instance, smoking, having open flames or using sparking tools to open or close storage containers should be prohibited. It is also advised that grounded and bonded metal containers are used for the transport of chloroprene.[19]
Occupational exposure limits[20]
The official legal body that develops and enforces occupational exposure limits (OEL) in order to ensure workplace safety and health regulations is the Occupational Health and Safety Administration (OSHA) that works under the U.S. Department of Labor. OSHA's permissible exposure limits (PELs), a guideline for occupational exposures, were adopted from the 1968 threshold limit values (TLVs) of the American Conference of Governmental Industrial Hygienists (ACGIH).[21] Each year, the ACGIH publish their TLV and BEI booklet that provides updated information on "occupational exposure guidelines for more than 700 chemical substances and physical agents."[22] The scientific literature on certain chemical and physical exposures has evolved since 1968, therefore OSHA recognizes that their PELs may not guarantee worker health and safety. The National Institute for Occupational Health and Safety (NIOSH) under the U.S. Department of Health and Human Services compensates for the rigidity of the PEL by researching "all medical, biological, engineering, chemical, and trade information relevant to the hazard" and publishing recommended exposure limits (RELs) based on their research.[21] Therefore, as a way to ensure worker safety and health, the following sections on safety guidelines and hazard control will consider the most recent occupational exposure limits from ACGIH's 2018 TLV and BEI booklet and NIOSH's REL.
A table of occupational exposure limits (OELs) from various jurisdictions follows. In general, the OELs range from 0.55 ppm to 25 ppm.[23]
| Occupational Exposure Limits for Chloroprene[23] | |
|---|---|
| Organization | Concentration |
| NIOSH REL | 1 ppm |
| ACGIH TLV 8-hour TWA | 1 ppm |
| OSHA PEL 8-hour TWA | 25 ppm |
| Mine Safety and Health Administration | 25 ppm |
| Austria OEL MAK-TMW | 5 ppm |
| Belgium OEL TWA | 10 ppm |
| Denmark OEL ceiling concentration | 1 ppm |
| Finland OEL TWA | 1 ppm |
| France OEL VME | 10 ppm |
| Hungary OEL TWA | 5 ppm |
| Iceland OEL Short Term Exposure Limit (STEL) | 1 ppm |
| Korea OEL TWA | 10 ppm |
| Mexico OEL TWA | 10 ppm |
| New Zealand OEL TWA | 10 ppm |
| Norway OEL TWA | 1 ppm |
| Peru OEL TWA | 10 ppm |
| Poland OEL MAC TWA | 0.55 ppm |
| Russia OEL STEL | 0.55 ppm |
| Sweden OEL TWA | 1 ppm |
| Switzerland OEL MAK-week | 5 ppm |
| The Netherlands OEL MAC-TGG | 5 ppm |
In the ACGIH's 2018 TLV and BEI booklet, chloroprene was designated with a skin and an A2 notation. The skin notation designation is based on animal and human research that have shown chloroprene's ability to be absorbed by the skin.[24] An A2 designation by the ACGIH means that the substance is a suspected human carcinogen with support from human data that are accepted as adequate in quality but may not be enough to declare an A1 (known human carcinogen) designation. Additionally, the TLV basis for these designations are due to scientific studies that show an association between chloroprene exposure and lung cancer, upper respiratory tract (URT) and eye irritation.[20]
Public health implications
Since chloroprene usage is limited to those facilities producing Neoprene, the occupational health risks are isolated to those facilities. However, insufficient control of chloroprene emissions may extend the health and safety concerns of chloroprene beyond the facility and into the surrounding areas. Chloroprene release is predominately as an air pollutant, but other feasible fates and transport of chloroprene in the environment are discussed below.
In the fourteenth edition of the National Institute of Health report on carcinogens, the half-life time differences between chloroprene in air, water and soil were highlighted. In the air, chloroprene “reacts with photo-chemically generated hydroxyl radicals” and has a half-life of 18 hours. The smaller amounts that are removed by reaction with ozone have a half-life of 10 days. In streams, chloroprene is stated to volatilize quickly with a half-life of 3 hours. However, in bigger bodies of water such as a lake, the half-life of chloroprene is 4 days. Similar to its reaction with water, chloroprene on soil was cited to volatilize from the surface. However, the report remarked that chloroprene holds the potential to leach into groundwater supplies.[25] Due to its volatility and extreme reactivity, the threat of chloroprene exists predominantly as an air pollutant and is not expected to bioaccumulate or persist in the environment according to the U.S EPA Toxicological Review of Chloroprene.[12] However, the Centers for Disease Control and Prevention (CDC) states that chloroprene does, in fact, have the potential to persist in the environment. Nonetheless, the primary route of exposure for animals and humans is inhalation, but can be absorbed through the skin or indigestion.[23]
In December 2015, the EPA released its 2011 National Air Toxic Assessment to help state and local agencies prioritize the required steps in identifying and mitigating sources of air pollution.[26] In this report, it was measured that chloroprene was being released from Denka Performance Elastomer's Pontchartrain facility located in LaPlace, Louisiana. EPA worked with the Louisiana Department of Environmental Quality, DuPont and the nonprofit organization Louisiana Environmental Action Network to institute monitoring of chloroprene pollution near the facility and in the surrounding neighborhood. Air monitoring is ongoing.
References
- NIOSH Pocket Guide to Chemical Hazards. "#0133". National Institute for Occupational Safety and Health (NIOSH).
- "ß-Chloroprene". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- Manfred Rossberg, Wilhelm Lendle, Gerhard Pfleiderer, Adolf Tögel, Eberhard-Ludwig Dreher, Ernst Langer, Heinz Rassaerts, Peter Kleinschmidt, Heinz Strack, Richard Cook, Uwe Beck, Karl-August Lipper, Theodore R. Torkelson, Eckhard Löser, Klaus K. Beutel, "Chlorinated Hydrocarbons" in Ullmann’s Encyclopedia of Industrial Chemistry, 2006 John Wiley-VCH: Weinheim. doi:10.1002/14356007.a06_233.pub2
- Carothers, Wallace H.; Williams, Ira.; Collins, Arnold M.; Kirby, James E. (November 1931). "Acetylene Polymers and their Derivatives. II. A New Synthetic Rubber: Chloroprene and its Polymers". Journal of the American Chemical Society. 53 (11): 4203–4225. doi:10.1021/ja01362a042.
- Smith, John K. (January 1985). "The Ten-Year Invention: Neoprene and Du Pont Research, 1930-1939". Technology and Culture. 26 (1): 34–55. doi:10.2307/3104528. JSTOR 3104528.
- "Hazard Communication Pictograms". U.S. Occupational Safety and Health Administration. Retrieved 2018-12-11.
- "OSHA Quick Card: Hazard Communication Standard Pictogram". U.S. Occupational Safety and Health Administration. Retrieved 2018-12-11.
- Pubchem. "Chloroprene". pubchem.ncbi.nlm.nih.gov. Retrieved 2018-11-24.
- "Flammable liquids" (PDF). www.osha.gov. Retrieved 2018-12-14.
- "National Fire Protection Association (NFPA) Rating System" (PDF). www.fm.colostate.edu. Retrieved 2018-12-14.
- "Chloroprene, Stabilized | Cameo Chemicals | NOAA". cameochemicals.noaa.gov. Retrieved 2018-12-14.
- U.S. EPA. IRIS Toxicological Review of Chloroprene (Final Report). U.S. Environmental Protection Agency, Washington, DC, EPA/635/R-09/010F, 2010.
- "Current intelligence bulletin 1 - chloroprene". 2018-10-16. doi:10.26616/NIOSHPUB781271. Cite journal requires
|journal=(help) - "Guidance for Hazard Determination for Compliance with the OSHA Hazard Communication Standard | Occupational Safety and Health Administration". www.osha.gov. Retrieved 2018-12-14.
- "Understanding REACH - ECHA". echa.europa.eu. Retrieved 2018-12-14.
- "The IRIS Review Process: Chloroprene and the Criticality of Good Science". S2CID 33811055. Cite journal requires
|journal=(help) - Lynch, Jeremiah (2001-06-01). "Occupational exposure to butadiene, isoprene and chloroprene". Chemico-Biological Interactions. 135–136: 207–214. doi:10.1016/S0009-2797(01)00191-0. ISSN 0009-2797. PMID 11397391.
- Rickert, Annette; Hartung, Benno; Kardel, Bernd; Teloh, Johanna; Daldrup, Thomas (2012-02-10). "A fatal intoxication by chloroprene". Forensic Science International. 215 (1–3): 110–113. doi:10.1016/j.forsciint.2011.03.029. ISSN 0379-0738. PMID 21511420.
- "Hazardous Substance Fact Sheet" (PDF). New Jersey Department of Health and Senior Services.
- 2018 TLVs and BEIs: Based on the Documentation of the Threshold Limit Values for Chemical Substances and Physical Agents & Biological Exposure Indices. ACGIH, 2018.
- "OSHA Annotated PELs | Occupational Safety and Health Administration". www.osha.gov. Retrieved 2018-11-25.
- "Product: *2018 TLVs and BEIs: ACGIH". www.acgih.org. Retrieved 2018-11-25.
- "Template Package 4". Centers for Disease Control and Prevention. Retrieved 2018-11-24.
- Lowry, Larry K. (2004). "Definitions and Interpretations of Skin Notations and the Use of Biological Monitoring to Assess Total Exposure" (PDF). ACGIH.
- National Institute of Environmental Health Sciences. National Toxicology Program. Report on carcinogens, fourteenth edition 2016. ISBN 9781523108527. OCLC 990561140.
- Environmental Protection Agency. “Action Plan.” Denka Performance Elastomer – Pontchartrain facility, June 2016.
External links
- International Chemical Safety Card 0133
- NIOSH Pocket Guide to Chemical Hazards. "#0133". National Institute for Occupational Safety and Health (NIOSH).
- IARC Monograph "Chloroprene."
- LaPlace, St. John the Baptist Parish, Louisiana
- Safety and Health Topics | Fire Safety | Occupational Safety and Health Administration