Cordycepin
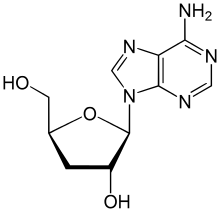
Cordycepin, or 3'-deoxyadenosine, is a derivative of the nucleoside adenosine, differing from the latter by the absence of the hydroxy group in the 3' position of its ribose moiety. It was initially extracted from the fungus Cordyceps militaris,[1] but can now be produced synthetically. It is also found in other Cordyceps species as well as Ophiocordyceps sinensis.[2]
 | |
| Names | |
|---|---|
| IUPAC name
9-(3-Deoxy-β-d-ribofuranosyl)adenine | |
| Other names
Cordycepine 3'-Deoxyadenosine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.720 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H13N5O3 | |
| Molar mass | 251.246 g·mol−1 |
| Melting point | 225.5 °C (437.9 °F; 498.6 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Because cordycepin is similar to adenosine, some enzymes cannot discriminate between the two. It can therefore participate in certain biochemical reactions (for example, incorporation into an mRNA molecule, resulting in premature termination of protein synthesis).[3][4]
Cordycepin has displayed cytotoxicity against some leukemic cell lines in vitro, and at least one clinical trial of cordycepin as a leukemia treatment is in progress.[5]
Cordycepin has been found to produce rapid, robust imipramine-like antidepressant effects in animal models of depression, and these effects, similarly to those of imipramine, are dependent on enhancement of AMPA receptor signaling.[6]
See also
References
- Cunningham, K. G.; Manson, W.; Spring, F. S. & Hutchinson, S. A. (1950). "Cordycepin, a Metabolic Product isolated from Cultures of Cordyceps militaris (Linn.) Link". Nature. 166 (4231): 949. Bibcode:1950Natur.166..949C. doi:10.1038/166949a0. PMID 14796634.
- Zhou, X; Luo, L; Dressel, W; Shadier, G; Krumbiegel, D; Schmidtke, P; Zepp, F; Meyer, CU (2008). "Cordycepin is an immunoregulatory active ingredient of Cordyceps sinensis". The American Journal of Chinese Medicine. 36 (5): 967–80. doi:10.1142/S0192415X08006387. PMID 19051361.
- Siev, M.; Weinberg, R. & Penman, S. (1969). "The selective interruption of nucleolar RNA synthesis in HeLa cells by cordycepin". J. Cell Biol. 41 (2): 510–520. doi:10.1083/jcb.41.2.510. PMC 2107749. PMID 5783871.
- Kondrashov A, Meijer HA, Barthet-Barateig A, Parker HN, Khurshid A, Tessier S, et al. (2012). "Inhibition of polyadenylation reduces inflammatory gene induction". RNA. 18 (12): 2236–50. doi:10.1261/rna.032391.112. PMC 3504674. PMID 23118416.
- National Cancer Institute (2011-02-02). "Definition of cordycepin". NCI Drug Dictionary. Retrieved 21 December 2015.
- Li, Bai; Hou, Yangyang; Zhu, Ming; Bao, Hongkun; Nie, Jun; Zhang, Grace Y.; Shan, Liping; Yao, Yao; Du, Kai; Yang, Hongju; Li, Meizhang; Zheng, Bingrong; Xu, Xiufeng; Xiao, Chunjie; Du, Jing (2016). "3'-Deoxyadenosine (Cordycepin) Produces a Rapid and Robust Antidepressant Effect via Enhancing Prefrontal AMPA Receptor Signaling Pathway". International Journal of Neuropsychopharmacology. 19 (4): pyv112. doi:10.1093/ijnp/pyv112. ISSN 1461-1457. PMC 4851261. PMID 26443809.
