Crystal twinning
Crystal twinning occurs when two separate crystals share some of the same crystal lattice points in a symmetrical manner. The result is an intergrowth of two separate crystals in a variety of specific configurations. The surface along which the lattice points are shared in twinned crystals is called a composition surface or twin plane.[1]
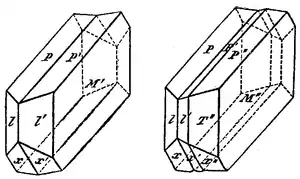
Crystallographers classify twinned crystals by a number of twin laws. These twin laws are specific to the crystal system. The type of twinning can be a diagnostic tool in mineral identification. Twinning is an important mechanism for permanent shape changes in a crystal.[2]
Twinning can often be a problem in X-ray crystallography, as a twinned crystal does not produce a simple diffraction pattern.
Twin laws
Twin laws are either defined by their twin planes (i.e. {hkl}) or the direction of the twin axes (i.e. [hkl]). If the twin law can be defined by a simple planar composition surface, the twin plane is always parallel to a possible crystal face and never parallel to an existing plane of symmetry (remember that twinning adds symmetry).
If the twin law is a rotation axis, the composition surface will be irregular, the twin axis will be perpendicular to a lattice plane, but will never be an even-fold rotation axis of the existing symmetry. For example, twinning cannot occur on a new 2-fold axis that is parallel to an existing 4-fold axis.[3]
Common twin laws
In the isometric system, the most common types of twins are the Spinel Law (twin plane, parallel to an octahedron) [111], where the twin axis is perpendicular to an octahedral face, and the Iron Cross [001], which is the interpenetration of two pyritohedrons, a subtype of dodecahedron.
In the hexagonal system, calcite shows the contact twin laws {0001} and {0112}. Quartz shows the Brazil Law {1120}, and Dauphiné Law [0001], which are penetration twins caused by transformation, and Japan Law {1122}, which is often caused by accidents during growth.
In the tetragonal system, cyclical contact twins are the most commonly observed type of twin, such as in rutile titanium dioxide and cassiterite tin oxide.
In the orthorhombic system, crystals usually twin on planes parallel to the prism face, where the most common is a {110} twin, which produces cyclical twins, such as in aragonite, chrysoberyl, and cerussite.
In the monoclinic system, twins occur most often on the planes {100} and {001} by the Manebach Law {001}, Carlsbad Law [001], Braveno Law {021} in orthoclase, and the Swallow Tail Twins {001} in gypsum.
In the triclinic system, the most commonly twinned crystals are the feldspar minerals plagioclase and microcline. These minerals show the Albite and Pericline Laws.[3]
Types of twinning


Simple twinned crystals may be contact twins or penetration twins. Contact twins share a single composition surface, often appearing as mirror images across the boundary. Plagioclase, quartz, gypsum, and spinel often exhibit contact twinning. Merohedral twinning occurs when the lattices of the contact twins superimpose in three dimensions, such as by relative rotation of one twin from the other. An example is metazeunerite. In penetration twins the individual crystals have the appearance of passing through each other in a symmetrical manner. Orthoclase, staurolite, pyrite, and fluorite often show penetration twinning.

If several twin crystal parts are aligned by the same twin law they are referred to as multiple or repeated twins. If these multiple twins are aligned in parallel they are called polysynthetic twins. When the multiple twins are not parallel they are cyclic twins. Albite, calcite, and pyrite often show polysynthetic twinning. Closely spaced polysynthetic twinning is often observed as striations or fine parallel lines on the crystal face. Rutile, aragonite, cerussite, and chrysoberyl often exhibit cyclic twinning, typically in a radiating pattern. But in general, based on the relationship between the twin axis and twin plane, there are 3 types of twinning:
- parallel twinning, when the twin axis and compositional plane lie parallel to each other,
- normal twinning, when the twin plane and compositional plane lie normally, and
- complex twinning, a combination of parallel twinning and normal twinning on one compositional plane.
Modes of formation
There are three modes of formation of twinned crystals. Growth twins are the result of an interruption or change in the lattice during formation or growth due to a possible deformation from a larger substituting ion. Annealing or transformation twins are the result of a change in crystal system during cooling as one form becomes unstable and the crystal structure must re-organize or transform into another more stable form. Deformation or gliding twins are the result of stress on the crystal after the crystal has formed. If a metal with face-centered cubic (fcc) structure, like Al, Cu, Ag, Au, etc., is subjected to stress, it will experience twinning. The formation and migration of twin boundaries is partly responsible for ductility and malleability of fcc metals.[4]
Deformation twinning is a common result of regional metamorphism. Crystal twinning is also used as an indicator of force direction in mountain building processes in orogeny research.
Crystals that grow adjacent to each other may be aligned to resemble twinning. This parallel growth simply reduces system energy and is not twinning.
Mechanisms of formation
Twinning can occur by cooperative displacement of atoms along the face of the twin boundary. This displacement of a large quantity of atoms simultaneously requires significant energy to perform. Therefore, the theoretical stress required to form a twin is quite high. It is believed that twinning is associated with dislocation motion on a coordinated scale, in contrast to slip, which is caused by independent glide at several locations in the crystal.
Twinning and slip are competitive mechanisms for crystal deformation. Each mechanism is dominant in certain crystal systems and under certain conditions. In fcc metals, slip is almost always dominant because the stress required is far less than twinning stress.
Compared to slip, twinning produces a deformation pattern that is more heterogeneous in nature. This deformation produces a local gradient across the material and near intersections between twins and grain boundaries. The deformation gradient can lead to fracture along the boundaries, particularly in bcc transition metals at low temperatures.
Deposition of twins
The conditions of crystal formation in solution have an effect on the type and density of dislocations in the crystal. It frequently happens that the crystal is oriented so that there will a more rapid deposition of material on one part than on another; for instance, if the crystal be attached to some other solid it cannot grow in that direction. If the crystal is freely suspended in the solution and material for growth is supplied at the same rate on all sides does an equably developed form result.[1]
Twin boundaries
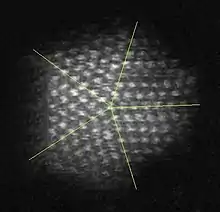
Twin boundaries occur when two crystals of the same type intergrow so that only a slight misorientation exists between them. It is a highly symmetrical interface, often with one crystal the mirror image of the other; also, atoms are shared by the two crystals at regular intervals. This is also a much lower-energy interface than the grain boundaries that form when crystals of arbitrary orientation grow together. Twin boundaries may also display a higher degree of symmetry than the single crystal. These twins are called mimetic or pseudo-symmetric twins.[1]
Twin boundaries are partly responsible for shock hardening and for many of the changes that occur in cold work of metals with limited slip systems or at very low temperatures. They also occur due to martensitic transformations: the motion of twin boundaries is responsible for the pseudoelastic and shape-memory behavior of nitinol, and their presence is partly responsible for the hardness due to quenching of steel. In certain types of high strength steels, very fine deformation twins act as primary obstacles against dislocation motion. These steels are referred to as 'TWIP' steels, where TWIP stands for twinning-induced plasticity.[5]
Appearance in different structures
Of the three common crystalline structures bcc, fcc, and hcp, the hcp structure is the most likely to form deformation twins when strained, because they rarely have a sufficient number of slip systems for an arbitrary shape change. High strain rates, low stacking-fault energy and low temperatures facilitate deformation twinning.[2]
References
- Spencer, Leonard James (1911). . Encyclopædia Britannica. 7 (11th ed.). pp. 569–591.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - Courtney, Thomas H. (2000) Mechanical Behavior of Materials, 2nd ed. McGraw Hill. ISBN 1-57766-425-6
- Nelson, Stephen A. (2013) Twinning, Polymorphism, Polytypism, Pseudomorphism. Tulane University
- Che Lah, Nurul Akmal and Trigueros, Sonia (2019). "Synthesis and modelling of the mechanical properties of Ag, Au and Cu nanowires". Sci. Technol. Adv. Mater. 20 (1): 225–261. doi:10.1080/14686996.2019.1585145. PMC 6442207. PMID 30956731.CS1 maint: multiple names: authors list (link)
- Steinmetz, D.R.; Jäpel, T.; Wietbrock, B.; Eisenlohr, P.; Gutierrez-Urrutia, I.; Saeed (2013), "Revealing the strain-hardening behavior of twinning-induced plasticity steels: Theory, simulations, experiments", Acta Materialia, 61 (2): 494, doi:10.1016/j.actamat.2012.09.064.
Further reading
- Hurlbut, Cornelius S.; Klein, Cornelis, 1985, Manual of Mineralogy, 20th ed., ISBN 0-471-80580-7