Decamethyltitanocene dichloride
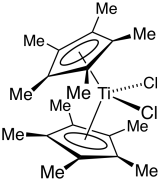
Decamethyltitanocene dichloride is an organotitanium compound with the formula Cp*2TiCl2 (where Cp* is C5(CH3)5, derived from pentamethylcyclopentadiene). It is a red solid that is soluble in nonpolar organic solvents. The complex has been the subject of extensive research. It is a precursor to many other organotitanium complexes
 | |
 | |
| Names | |
|---|---|
| Other names
Bis(Pentamethylcyclopentadienyl)titanium dichloride | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.149.726 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C20H30Cl2Ti | |
| Molar mass | 389.23 g·mol−1 |
| Appearance | red solid |
| Density | 1.32 g/cm3 |
| Melting point | 190 °C (374 °F; 463 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis and reactions
The complex is prepared from titanium tetrachloride with LiCp*. An intermediate in this synthesis is (pentamethylclopentadienyl)titanium trichloride.
Reduction of Cp*2TiCl2 in the presence of ethylene gives the adduct Cp*2Ti(C2H4).[1] The dicarbonyl complex Cp*2Ti(CO)2 is prepared similarly.[2]
Further reading
- Buchwald, S. L.; Nielsen, R. B. (1988). "Group 4 Metal Complexes of Benzynes, Cycloalkynes, Acyclic Alkynes, and Alkenes". Chemical Reviews. 88 (7): 1047–1058. doi:10.1021/cr00089a004.
- Rosenthal, U.; et al. (2000). "What Do Titano- and Zirconocenes Do with Diynes and Polyynes?". Chemical Reviews. 33: 119–129. doi:10.1021/ar9900109. PMID 10673320.
References
- Cohen, Steven A.; Auburn, Pamela R.; Bercaw, John E. (1983). "Structure and Reactivity of Bis(pentamethylcyclopentadienyl)(ethylene)titanium(II), a Simple Olefin Adduct of Titanium". Journal of the American Chemical Society. 105 (5): 1136–1143. doi:10.1021/ja00343a012.
- Sikora, David J.; Moriarty, Kevin J.; Rausch, Marvin D. (1990). Dicarbonylbis(η5 -Cyclopentadienyl) Complexes of Titanium, Zirconium, and Hafnium. Inorganic Syntheses. 28. pp. 248–257. doi:10.1002/9780470132593.ch64. ISBN 9780470132593.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.