Deep brain stimulation
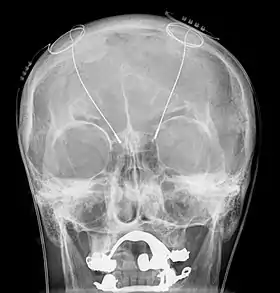
Deep brain stimulation (DBS) is a neurosurgical procedure involving the placement of a medical device called a neurostimulator (sometimes referred to as a "brain pacemaker"), which sends electrical impulses, through implanted electrodes, to specific targets in the brain (brain nuclei) for the treatment of movement disorders, including Parkinson's disease, essential tremor, and dystonia.[1] While its underlying principles and mechanisms are not fully understood, DBS directly changes brain activity in a controlled manner.[2][3]
| Deep brain stimulation | |
|---|---|
 | |
| MeSH | D046690 |
| MedlinePlus | 007453 |
DBS has been approved by the Food and Drug Administration as a treatment for essential tremor and Parkinson's disease (PD) since 1997.[4] DBS was approved for dystonia in 2003,[5] obsessive–compulsive disorder (OCD) in 2009, and epilepsy in 2018.[6][7][8] DBS has been studied in clinical trials as a potential treatment for chronic pain for various affective disorders, including major depression. It is one of few neurosurgical procedures that allow blinded studies.[1]
Medical use

Parkinson's disease
DBS is used to manage some of the symptoms of Parkinson's disease that cannot be adequately controlled with medications.[9][10] It is recommended for people who have PD with motor fluctuations and tremor inadequately controlled by medication, or to those who are intolerant to medication, as long as they do not have severe neuropsychiatric problems.[11] Four areas of the brain have been treated with neural stimulators in PD. These are the globus pallidus internus, thalamus, subthalamic nucleus and the pedunculopontine nucleus. However, most DBS surgeries in routine practice target either the globus pallidus internus, or the Subthalamic nucleus.
- DBS of the globus pallidus internus reduces uncontrollable shaking movements called dyskinesias. This enables a patient to take adequate quantities of medications (especially levodopa), thus leading to better control of symptoms.
- DBS of the subthalamic nucleus directly reduces symptoms of Parkinson's. This enables a decrease in the dose of anti-parkinonian medications.
- DBS of the PPN may help with freezing of gait, while DBS of the thalamus may help with tremor. These targets are not routinely utilized.
Selection of the correct DBS target is a complicated process. Multiple clinical characteristics are used to select the target including – identifying the most troublesome symptoms, the dose of levodopa that the patient is currently taking, the effects and side-effects of current medications and concurrent problems. For example, subthalamic nucleus DBS may worsen depression and hence is not preferred in patients with uncontrolled depression.
Generally DBS is associated with 30–60% improvement in motor score evaluations.[12]
Tourette syndrome
DBS has been used experimentally in treating adults with severe Tourette syndrome that does not respond to conventional treatment. Despite widely publicized early successes, DBS remains a highly experimental procedure for the treatment of Tourette's, and more study is needed to determine whether long-term benefits outweigh the risks.[13][14][15][16] The procedure is well tolerated, but complications include "short battery life, abrupt symptom worsening upon cessation of stimulation, hypomanic or manic conversion, and the significant time and effort involved in optimizing stimulation parameters".[17] As of 2006, five people with TS had been reported on; all experienced reduction in tics and the disappearance of obsessive-compulsive behaviors.[17]
The procedure is invasive and expensive, and requires long-term expert care. Benefits for severe Tourette's are not conclusive, considering less robust effects of this surgery seen in the Netherlands. Tourette's is more common in pediatric populations, tending to remit in adulthood, so in general this would not be a recommended procedure for use on children. Because diagnosis of Tourette's is made based on a history of symptoms rather than analysis of neurological activity, it may not always be clear how to apply DBS for a particular person. Due to concern over the use of DBS in Tourette syndrome treatment, the Tourette Association of America convened a group of experts to develop recommendations guiding the use and potential clinical trials of DBS for TS.[18]
Robertson reported that DBS had been used on 55 adults by 2011, remained an experimental treatment at that time, and recommended that the procedure "should only be conducted by experienced functional neurosurgeons operating in centres which also have a dedicated Tourette syndrome clinic".[14] According to Malone et al. (2006), "Only patients with severe, debilitating, and treatment-refractory illness should be considered; while those with severe personality disorders and substance-abuse problems should be excluded."[17] Du et al. (2010) say, "As an invasive therapy, DBS is currently only advisable for severely affected, treatment-refractory TS adults".[15] Singer (2011) says, "pending determination of patient selection criteria and the outcome of carefully controlled clinical trials, a cautious approach is recommended".[13] Viswanathan et al. (2012) say DBS should be used for people with "severe functional impairment that cannot be managed medically".[19]
Adverse effects
DBS carries the risks of major surgery, with a complication rate related to the experience of the surgical team. The major complications include hemorrhage (1–2%) and infection (3–5%).[20]
The potential exists for neuropsychiatric side effects after DBS, including apathy, hallucinations, hypersexuality, cognitive dysfunction, depression, and euphoria. However, these may be temporary and related to correct placement of electrodes and calibration of the stimulator, so these side effects are potentially reversible.[21]
Because the brain can shift slightly during surgery, the electrodes can become displaced or dislodged from the specific location. This may cause more profound complications such as personality changes, but electrode misplacement is relatively easy to identify using CT scan. Also, complications of surgery may occur, such as bleeding within the brain. After surgery, swelling of the brain tissue, mild disorientation, and sleepiness are normal. After 2–4 weeks, a follow-up visit is used to remove sutures, turn on the neurostimulator, and program it.
Impaired swimming skills surfaced as an unexpected risk of the procedure; several Parkinson's disease patients lost their ability to swim after receiving deep brain stimulation.[22][23]
Mechanisms
The exact mechanism of action of DBS is not known.[24] A variety of hypotheses try to explain the mechanisms of DBS:[25][26]
- Depolarization blockade: Electrical currents block the neuronal output at or near the electrode site.
- Synaptic inhibition: This causes an indirect regulation of the neuronal output by activating axon terminals with synaptic connections to neurons near the stimulating electrode.
- Desynchronization of abnormal oscillatory activity of neurons
- Antidromic activation either activating/blockading distant neurons or blockading slow axons[3]
DBS represents an advance on previous treatments which involved pallidotomy (i.e., surgical ablation of the globus pallidus) or thalamotomy (i.e., surgical ablation of the thalamus).[27] Instead, a thin lead with multiple electrodes is implanted in the globus pallidus, nucleus ventralis intermedius thalami, or subthalamic nucleus, and electric pulses are used therapeutically. The lead from the implant is extended to the neurostimulator under the skin in the chest area.
Its direct effect on the physiology of brain cells and neurotransmitters is currently debated, but by sending high-frequency electrical impulses into specific areas of the brain, it can mitigate symptoms[28] and directly diminish the side effects induced by PD medications,[29] allowing a decrease in medications, or making a medication regimen more tolerable.
Components and placement
The DBS system consists of three components: the implanted pulse generator (IPG), the lead, and an extension. The IPG is a battery-powered neurostimulator encased in a titanium housing, which sends electrical pulses to the brain that interfere with neural activity at the target site. The lead is a coiled wire insulated in polyurethane with four platinum-iridium electrodes and is placed in one or two different nuclei of the brain. The lead is connected to the IPG by an extension, an insulated wire that runs below the skin, from the head, down the side of the neck, behind the ear, to the IPG, which is placed subcutaneously below the clavicle, or in some cases, the abdomen.[9] The IPG can be calibrated by a neurologist, nurse, or trained technician to optimize symptom suppression and control side effects.[30]
DBS leads are placed in the brain according to the type of symptoms to be addressed. For non-Parkinsonian essential tremor, the lead is placed in either the ventrointermediate nucleus of the thalamus or the zona incerta;[31] for dystonia and symptoms associated with PD (rigidity, bradykinesia/akinesia, and tremor), the lead may be placed in either the globus pallidus internus or the subthalamic nucleus; for OCD and depression to the nucleus accumbens; for incessant pain to the posterior thalamic region or periaqueductal gray; and for epilepsy treatment to the anterior thalamic nucleus.[32]
All three components are surgically implanted inside the body. Lead implantation may take place under local anesthesia or under general anesthesia ("asleep DBS") such as for dystonia. A hole about 14 mm in diameter is drilled in the skull and the probe electrode is inserted stereotactically, using either frame-based or frameless stereotaxis.[33] During the awake procedure with local anesthesia, feedback from the person is used to determine the optimal placement of the permanent electrode. During the asleep procedure, intraoperative MRI guidance is used for direct visualization of brain tissue and device.[34] The installation of the IPG and extension leads occurs under general anesthesia.[35] The right side of the brain is stimulated to address symptoms on the left side of the body and vice versa.
Research
Chronic pain
Stimulation of the periaqueductal gray and periventricular gray for nociceptive pain, and the internal capsule, ventral posterolateral nucleus, and ventral posteromedial nucleus for neuropathic pain has produced impressive results with some people, but results vary. One study[36] of 17 people with intractable cancer pain found that 13 were virtually pain free and only four required opioid analgesics on release from hospital after the intervention. Most ultimately did resort to opioids, usually in the last few weeks of life.[37] DBS has also been applied for phantom limb pain.[38]
Major depression and obsessive-compulsive disorder

DBS has been used in a small number of clinical trials to treat people with severe treatment-resistant depression (TRD).[39] A number of neuroanatomical targets have been used for DBS for TRD including the subgenual cingulate gyrus, posterior gyrus rectus,[40] nucleus accumbens,[41] ventral capsule/ventral striatum, inferior thalamic peduncle, and the lateral habenula.[39] A recently proposed target of DBS intervention in depression is the superolateral branch of the medial forebrain bundle; its stimulation lead to surprisingly rapid antidepressant effects.[42]
The small numbers in the early trials of DBS for TRD currently limit the selection of an optimal neuroanatomical target.[39] Evidence is insufficient to support DBS as a therapeutic modality for depression; however, the procedure may be an effective treatment modality in the future.[43] In fact, beneficial results have been documented in the neurosurgical literature, including a few instances in which people who were deeply depressed were provided with portable stimulators for self treatment.[44][45][46]
A systematic review of DBS for TRD and OCD identified 23 cases, nine for OCD, seven for TRD, and one for both. "[A]bout half the patients did show dramatic improvement" and adverse events were "generally trivial" given the younger age of the psychiatric population relative to the age of people with movement disorders.[47] The first randomized, controlled study of DBS for the treatment of TRD targeting the ventral capsule/ventral striatum area did not demonstrate a significant difference in response rates between the active and sham groups at the end of a 16-week study.[48] However, a second randomized controlled study of ventral capsule DBS for TRD did demonstrate a significant difference in response rates between active DBS (44% responders) and sham DBS (0% responders).[49] Efficacy of DBS is established for OCD, with on average 60% responders in severely ill and treatment-resistant patients.[50] Based on these results the FDA has approved DBS for treatment-resistant OCD under a Humanitarian Device Exemption (HDE), requiring that the procedure be performed only in a hospital with specialist qualifications to do so.
DBS for TRD can be as effective as antidepressants, with good response and remission rates, but adverse effects and safety must be more fully evaluated. Common side effects include "wound infection, perioperative headache, and worsening/irritable mood [and] increased suicidality".[51]
Other clinical applications
Results of DBS in people with dystonia, where positive effects often appear gradually over a period of weeks to months, indicate a role of functional reorganization in at least some cases.[52] The procedure has been tested for effectiveness in people with epilepsy that is resistant to medication.[53] DBS may reduce or eliminate epileptic seizures with programmed or responsive stimulation.
DBS of the septal areas of persons with schizophrenia have resulted in enhanced alertness, cooperation, and euphoria.[54] Persons with narcolepsy and complex-partial seizures also reported euphoria and sexual thoughts from self-elicited DBS of the septal nuclei.[45]
Orgasmic ecstasy was reported with the electrical stimulation of the brain with depth electrodes in the left hippocampus at 3mA, and the right hippocampus at 1 mA.[55]
In 2015, a group of Brazilian researchers led by neurosurgeon Erich Fonoff described a new technique that allows for simultaneous implants of electrodes called bilateral stereotactic procedure for DBS. The main benefits are less time spent on the procedure and greater accuracy.[56]
In 2016, DBS was found to improve learning and memory in a mouse model of Rett syndrome.[57] More recent (2018) work showed, that forniceal DBS upregulates genes involved in synaptic function, cell survival, and neurogenesis,[58] making some first steps at explaining the restoration of hippocampal circuit function.
See also
References
- Kringelbach ML, Jenkinson N, Owen SL, Aziz TZ (August 2007). "Translational principles of deep brain stimulation". Nature Reviews. Neuroscience. 8 (8): 623–35. doi:10.1038/nrn2196. PMID 17637800.
- Hammond C, Ammari R, Bioulac B, Garcia L (November 2008). "Latest view on the mechanism of action of deep brain stimulation". Movement Disorders. 23 (15): 2111–21. doi:10.1002/mds.22120. PMID 18785230. S2CID 14905206.
- García MR, Pearlmutter BA, Wellstead PE, Middleton RH (2013). "A slow axon antidromic blockade hypothesis for tremor reduction via deep brain stimulation". PLOS ONE. 8 (9): e73456. Bibcode:2013PLoSO...873456G. doi:10.1371/journal.pone.0073456. PMC 3774723. PMID 24066049.
- "Press Announcements - FDA approves brain implant to help reduce Parkinson's disease and essential tremor symptoms". FDA. Retrieved May 23, 2016.
The first device, Medtronic’s Activa Deep Brain Stimulation Therapy System, was approved in 1997 for tremor associated with essential tremor and Parkinson’s disease.
- 'Brain pacemaker' treats dystonia. KNBC TV, April 22, 2003. Retrieved October 18, 2006.
- "Press Release | Newsroom | Medtronic | Medtronic Receives FDA Approval for Deep Brain Stimulation Therapy for Medically Refractory Epilepsy". newsroom.medtronic.com. Retrieved 2018-12-18.
- "FDA Approves Humanitarian Device Exemption for Deep Brain Stimulator for Severe Obsessive-Compulsive Disorder". FDA.
- Gildenberg PL (2005). "Evolution of neuromodulation". Stereotactic and Functional Neurosurgery. 83 (2–3): 71–9. doi:10.1159/000086865. PMID 16006778.
- National Institute of Neurological Disorders and Stroke. Deep brain stimulation for Parkinson's disease information page Archived 2016-11-20 at the Wayback Machine Retrieved November 23, 2006.
- U.S. Department of Health and Human Services. FDA approves implanted brain stimulator to control tremors. Retrieved February 10, 2015.
- Bronstein JM, et al. (February 2011). "Deep brain stimulation for Parkinson disease: an expert consensus and review of key issues". Archives of Neurology. 68 (2): 165. doi:10.1001/archneurol.2010.260. PMC 4523130. PMID 20937936.
- Dallapiazza, R. F.; Vloo, P. D.; Fomenko, A.; Lee, D. J.; Hamani, C.; Munhoz, R. P.; Hodaie, M.; Lozano, A. M.; Fasano, A.; Kalia (2018). "Considerations for Patient and Target Selection in Deep Brain Stimulation surgery for Parkinson's disease". In Stoker, T. B.; Greenland, J. C. (eds.). Parkinson’s disease: Pathogenesis and clinical aspects. Brisbane: Codon Publications. doi:10.15586/codonpublications.parkinsonsdisease.2018.ch8. ISBN 978-0-9944381-6-4.
- Singer HS (March 2005). Tourette syndrome and other tic disorders. Handbook of Clinical Neurology. 100. pp. 641–57. doi:10.1016/B978-0-444-52014-2.00046-X. ISBN 9780444520142. PMID 21496613. Also see Singer HS (March 2005). "Tourette's syndrome: from behaviour to biology". The Lancet. Neurology. 4 (3): 149–59. doi:10.1016/S1474-4422(05)01012-4. PMID 15721825.
- Robertson MM (February 2011). "Gilles de la Tourette syndrome: the complexities of phenotype and treatment". British Journal of Hospital Medicine. 72 (2): 100–7. doi:10.12968/hmed.2011.72.2.100. PMID 21378617.
- Du JC, Chiu TF, Lee KM, Wu HL, Yang YC, Hsu SY, Sun CS, Hwang B, Leckman JF (October 2010). "Tourette syndrome in children: an updated review". Pediatrics and Neonatology. 51 (5): 255–64. doi:10.1016/S1875-9572(10)60050-2. PMID 20951354.
- Tourette Syndrome Association. Statement: Deep Brain Stimulation and Tourette Syndrome. Retrieved November 22, 2005.
- Malone DA, Pandya MM (2006). "Behavioral neurosurgery". Advances in Neurology. 99: 241–7. PMID 16536372.
- Mink JW, Walkup J, Frey KA, Como P, Cath D, Delong MR, Erenberg G, Jankovic J, Juncos J, Leckman JF, Swerdlow N, Visser-Vandewalle V, Vitek JL (November 2006). "Patient selection and assessment recommendations for deep brain stimulation in Tourette syndrome" (PDF). Movement Disorders. 21 (11): 1831–8. doi:10.1002/mds.21039. hdl:2027.42/55891. PMID 16991144.
- Viswanathan A, Jimenez-Shahed J, Baizabal Carvallo JF, Jankovic J (2012). "Deep brain stimulation for Tourette syndrome: target selection". Stereotactic and Functional Neurosurgery. 90 (4): 213–24. doi:10.1159/000337776. PMID 22699684.
- Doshi PK (April 2011). "Long-term surgical and hardware-related complications of deep brain stimulation". Stereotactic and Functional Neurosurgery. 89 (2): 89–95. doi:10.1159/000323372. PMID 21293168.
- Burn DJ, Tröster AI (September 2004). "Neuropsychiatric complications of medical and surgical therapies for Parkinson's disease". Journal of Geriatric Psychiatry and Neurology. 17 (3): 172–80. doi:10.1177/0891988704267466. PMID 15312281.
- "Deep Brain Stimulation May Put Parkinson's Patients at Risk for Drowning". www.medpagetoday.com. 2019-11-27. Retrieved 2019-12-09.
- Bangash, Omar K.; Thorburn, Megan; Garcia-Vega, Jimena; Walters, Susan; Stell, Rick; Starkstein, Sergio E.; Lind, Christopher R. P. (May 2016). "Drowning hazard with deep brain stimulation: case report". Journal of Neurosurgery. 124 (5): 1513–1516. doi:10.3171/2015.5.JNS15589. ISSN 1933-0693. PMID 26566200.
- Mogilner A.Y.; Benabid A.L.; Rezai A.R. (2004). "Chronic Therapeutic Brain Stimulation: History, Current Clinical Indications, and Future Prospects". In Markov, Marko; Paul J. Rosch (eds.). Bioelectromagnetic medicine. New York, N.Y: Marcel Dekker. pp. 133–51. ISBN 978-0-8247-4700-8.
- McIntyre CC, Thakor NV (2002). "Uncovering the mechanisms of deep brain stimulation for Parkinson's disease through functional imaging, neural recording, and neural modeling". Critical Reviews in Biomedical Engineering. 30 (4–6): 249–81. doi:10.1615/critrevbiomedeng.v30.i456.20. PMID 12739751.
- Herrington TM, Cheng JJ, Eskandar EN (January 2016). "Mechanisms of deep brain stimulation". Journal of Neurophysiology. 115 (1): 19–38. doi:10.1152/jn.00281.2015. PMC 4760496. PMID 26510756.
- Machado A, Rezai AR, Kopell BH, Gross RE, Sharan AD, Benabid AL (June 2006). "Deep brain stimulation for Parkinson's disease: surgical technique and perioperative management". Movement Disorders. 21 Suppl 14 (Suppl 14): S247–58. doi:10.1002/mds.20959. PMID 16810722. S2CID 18194178.
- Moro E, Lang AE (November 2006). "Criteria for deep-brain stimulation in Parkinson's disease: review and analysis". Expert Review of Neurotherapeutics. 6 (11): 1695–705. doi:10.1586/14737175.6.11.1695. PMID 17144783.
- Apetauerova D, Ryan RK, Ro SI, Arle J, Shils J, Papavassiliou E, Tarsy D (August 2006). "End of day dyskinesia in advanced Parkinson's disease can be eliminated by bilateral subthalamic nucleus or globus pallidus deep brain stimulation". Movement Disorders. 21 (8): 1277–9. doi:10.1002/mds.20896. PMID 16637040.
- Volkmann J, Herzog J, Kopper F, Deuschl G (2002). "Introduction to the programming of deep brain stimulators". Movement Disorders. 17 Suppl 3: S181–7. doi:10.1002/mds.10162. PMID 11948775.
- Lee JY, Deogaonkar M, Rezai A (July 2007). "Deep brain stimulation of globus pallidus internus for dystonia". Parkinsonism & Related Disorders. 13 (5): 261–5. doi:10.1016/j.parkreldis.2006.07.020. PMID 17081796.
- Deep brain stimulation. Surgery Encyclopedia. Retrieved January 25, 2007.
- Owen CM, Lindsey ME (May 2009). "Frame-based stereotaxy in a frameless era: current capabilities, relative role, and the positive- and negative predictive values of blood through the needle". Journal of Neuro-Oncology. 93 (1): 139–149. doi:10.1007/s11060-009-9871-y. PMID 19430891.
- Starr PA, Martin AJ, Ostrem JL, Talke P, Levesque N, Larson PS (March 2010). "Subthalamic nucleus deep brain stimulator placement using high-field interventional magnetic resonance imaging and a skull-mounted aiming device: technique and application accuracy". Journal of Neurosurgery. 112 (3): 479–90. doi:10.3171/2009.6.JNS081161. PMC 2866526. PMID 19681683.
- Deep Brain Stimulation, Department of Neurological Surgery, University of Pittsburgh. Retrieved May 13, 2008.
- Electrical stimulation of the brain for relief of intractable pain due to cancer. Cancer. 1986;57:1266–72. doi:10.1002/1097-0142(19860315)57:6<1266::aid-cncr2820570634>3.0.co;2-q. PMID 3484665.
- Johnson MI, Oxberry SG & Robb K. Stimulation-induced analgesia. In: Sykes N, Bennett MI & Yuan C-S. Clinical pain management: Cancer pain. 2nd ed. London: Hodder Arnold; 2008. ISBN 978-0-340-94007-5. p. 235–250.
- Kringelbach ML, Jenkinson N, Green AL, Owen SL, Hansen PC, Cornelissen PL, Holliday IE, Stein J, Aziz TZ (February 2007). "Deep brain stimulation for chronic pain investigated with magnetoencephalography". NeuroReport. 18 (3): 223–8. CiteSeerX 10.1.1.511.2667. doi:10.1097/wnr.0b013e328010dc3d. PMID 17314661.
- Anderson RJ, Frye MA, Abulseoud OA, Lee KH, McGillivray JA, Berk M, Tye SJ (September 2012). "Deep brain stimulation for treatment-resistant depression: efficacy, safety and mechanisms of action". Neuroscience and Biobehavioral Reviews. 36 (8): 1920–33. doi:10.1016/j.neubiorev.2012.06.001. PMID 22721950.
- Accolla EA, Aust S, Merkl A, Schneider GH, Kühn AA, Bajbouj M, Draganski B (April 2016). "Deep brain stimulation of the posterior gyrus rectus region for treatment resistant depression". Journal of Affective Disorders. 194: 33–7. doi:10.1016/j.jad.2016.01.022. PMID 26802505.
- Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, Joe AY, Kreft M, Lenartz D, Sturm V (January 2008). "Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression". Neuropsychopharmacology. 33 (2): 368–77. doi:10.1038/sj.npp.1301408. PMID 17429407.
- Schlaepfer TE, Bewernick BH, Kayser S, Mädler B, Coenen VA (June 2013). "Rapid effects of deep brain stimulation for treatment-resistant major depression". Biological Psychiatry. 73 (12): 1204–12. doi:10.1016/j.biopsych.2013.01.034. PMID 23562618.
- Murphy, Destiny N.; Boggio, Paulo; Fregni, Felipe (2009). "Transcranial direct current stimulation as a therapeutic tool for the treatment of major depression: insights from past and recent clinical studies". Curr Opin Psychiatry. 22 (3): 306–11. doi:10.1097/YCO.0b013e32832a133f.
- Delgado, Jose (1986). Physical Control of the Mind: Toward a Psychocivilized Society. New York: Harper and Row. ISBN 0-06-131914-7.
- Faria MA (2013). "Violence, mental illness, and the brain - A brief history of psychosurgery: Part 3 - From deep brain stimulation to amygdalotomy for violent behavior, seizures, and pathological aggression in humans". Surgical Neurology International. 4 (1): 91. doi:10.4103/2152-7806.115162. PMC 3740620. PMID 23956934.
- Robison RA, Taghva A, Liu CY, Apuzzo ML (2012). "Surgery of the mind, mood, and conscious state: an idea in evolution". World Neurosurgery. 77 (5–6): 662–86. doi:10.1016/j.wneu.2012.03.005. PMID 22446082.
- Lakhan SE, Callaway E (March 2010). "Deep brain stimulation for obsessive-compulsive disorder and treatment-resistant depression: systematic review". BMC Research Notes. 3 (1): 60. doi:10.1186/1756-0500-3-60. PMC 2838907. PMID 20202203.
- Dougherty DD, Rezai AR, Carpenter LL, Howland RH, Bhati MT, O'Reardon JP, Eskandar EN, Baltuch GH, Machado AD, Kondziolka D, Cusin C, Evans KC, Price LH, Jacobs K, Pandya M, Denko T, Tyrka AR, Brelje T, Deckersbach T, Kubu C, Malone DA (August 2015). "A Randomized Sham-Controlled Trial of Deep Brain Stimulation of the Ventral Capsule/Ventral Striatum for Chronic Treatment-Resistant Depression". Biological Psychiatry. 78 (4): 240–8. doi:10.1016/j.biopsych.2014.11.023. PMID 25726497.
- Bergfeld IO, Mantione M, Hoogendoorn ML, Ruhé HG, Notten P, van Laarhoven J, et al. (May 2016). "Deep Brain Stimulation of the Ventral Anterior Limb of the Internal Capsule for Treatment-Resistant Depression: A Randomized Clinical Trial". JAMA Psychiatry. 73 (5): 456–64. doi:10.1001/jamapsychiatry.2016.0152. PMID 27049915.
- Alonso P, Cuadras D, Gabriëls L, Denys D, Goodman W, Greenberg BD, et al. (2015-07-24). "Deep Brain Stimulation for Obsessive-Compulsive Disorder: A Meta-Analysis of Treatment Outcome and Predictors of Response". PLOS ONE. 10 (7): e0133591. Bibcode:2015PLoSO..1033591A. doi:10.1371/journal.pone.0133591. PMC 4514753. PMID 26208305.
- Moreines JL, McClintock SM, Holtzheimer PE (January 2011). "Neuropsychologic effects of neuromodulation techniques for treatment-resistant depression: a review". Brain Stimulation. 4 (1): 17–27. doi:10.1016/j.brs.2010.01.005. PMC 3023999. PMID 21255751.
- Krauss JK (2002). "Deep brain stimulation for dystonia in adults. Overview and developments". Stereotactic and Functional Neurosurgery. 78 (3–4): 168–82. doi:10.1159/000068963. PMID 12652041.
- Wu C, Sharan AD (Jan–Feb 2013). "Neurostimulation for the treatment of epilepsy: a review of current surgical interventions". Neuromodulation. 16 (1): 10–24, discussion 24. doi:10.1111/j.1525-1403.2012.00501.x. PMID 22947069.
- Heath RG (January 1972). "Pleasure and brain activity in man. Deep and surface electroencephalograms during orgasm". The Journal of Nervous and Mental Disease. 154 (1): 3–18. doi:10.1097/00005053-197201000-00002. PMID 5007439.
- Surbeck W, Bouthillier A, Nguyen DK (2013). "Bilateral cortical representation of orgasmic ecstasy localized by depth electrodes". Epilepsy & Behavior Case Reports. 1: 62–5. doi:10.1016/j.ebcr.2013.03.002. PMC 4150648. PMID 25667829.
- Fonoff ET, Azevedo A, Angelos JS, Martinez RC, Navarro J, Reis PR, Sepulveda ME, Cury RG, Ghilardi MG, Teixeira MJ, Lopez WO (July 2016). "Simultaneous bilateral stereotactic procedure for deep brain stimulation implants: a significant step for reducing operation time". Journal of Neurosurgery. 125 (1): 85–9. doi:10.3171/2015.7.JNS151026. PMID 26684776.
- Lu H, Ash RT, He L, Kee SE, Wang W, Yu D, Hao S, Meng X, Ure K, Ito-Ishida A, Tang B, Sun Y, Ji D, Tang J, Arenkiel BR, Smirnakis SM, Zoghbi HY (August 2016). "Loss and Gain of MeCP2 Cause Similar Hippocampal Circuit Dysfunction that Is Rescued by Deep Brain Stimulation in a Rett Syndrome Mouse Model". Neuron. 91 (4): 739–747. doi:10.1016/j.neuron.2016.07.018. PMC 5019177. PMID 27499081.
- Pohodich AE, Yalamanchili H, Raman AT, Wan YW, Gundry M, Hao S, Jin H, Tang J, Liu Z, Zoghbi HY (March 2018). "Forniceal deep brain stimulation induces gene expression and splicing changes that promote neurogenesis and plasticity". eLife. 7. doi:10.7554/elife.34031. PMC 5906096. PMID 29570050.
Further reading
- Appleby BS, Duggan PS, Regenberg A, Rabins PV (September 2007). "Psychiatric and neuropsychiatric adverse events associated with deep brain stimulation: A meta-analysis of ten years' experience". Movement Disorders. 22 (12): 1722–8. doi:10.1002/mds.21551. PMID 17721929. S2CID 22925963.
- Schlaepfer TE, Bewernick BH, Kayser S, Hurlemann R, Coenen VA (May 2014). "Deep brain stimulation of the human reward system for major depression--rationale, outcomes and outlook". Neuropsychopharmacology. 39 (6): 1303–14. doi:10.1038/npp.2014.28. PMC 3988559. PMID 24513970.
- Diamond A, Shahed J, Azher S, Dat-Vuong K, Jankovic J (May 2006). "Globus pallidus deep brain stimulation in dystonia". Movement Disorders. 21 (5): 692–5. doi:10.1002/mds.20767. PMID 16342255. S2CID 29677149.
- Richter EO, Lozano AM (2004). "Deep Brain Stimulation for Parkinson's Disease in Movement Disorders". In Markov M, Rosch PJ (eds.). Bioelectromagnetic medicine. New York, N.Y: Marcel Dekker. pp. 265–76. ISBN 978-0-8247-4700-8.
External links
| Wikimedia Commons has media related to Deep brain stimulation. |
- Video: Deep brain stimulation to treat Parkinson's disease
- Video: Deep brain stimulation therapy for Parkinson's disease
- The Perils of Deep Brain Stimulation for Depression. Author Danielle Egan. September 24, 2015.
- Treatment center for Deep Brain Stimulation of movement disorders, OCD, Tourette or depression.
- Treatment center for Deep Brain Stimulation for OCD
