Desosamine
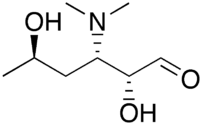
Desosamine is a 3-(dimethylamino)-3,4,6-trideoxyhexose found in certain macrolide antibiotics (contain a high level of microbial resistance) such as the commonly prescribed erythromycin,[1][2] azithromycin, clarithroymcin, methymycin, narbomycin, oleandomycin, picromycin and roxithromycin. As the name suggests, these macrolide antibiotics contain a macrolide or lactone ring and attached to the ring is desosamine which is crucial for bactericidal activity.[3]
 | |
| Names | |
|---|---|
| IUPAC name
(2R,3S,5R)-3-(Dimethylamino)-2,5-dihydroxyhexanal | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H17NO3 | |
| Molar mass | 175.23 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Biosynthesis
Six enzymes are required for its biosynthesis from TDP-glucose in Streptomyces venezuelae.[1] Desosamine serves as the sugar donor in this formation.
Degradation
Degradation of several of the aforementioned antibiotics yields the desosamine sugar. It is found in combination with the smaller macrolide rings, always attached at C-3 or C-5 of the aglycone. Alkaline degradation found the sugar to be a D-hexose derivative.[4] Glycosidic cleavage of methomycin produces aglycone methynolide and the basic sugar desosamine, whose structure had been determined by oxidative degradation to crotonaldehyde and by other experiments.[5]
References
- Rodríguez, Eduardo; Peirú, Salvador; Carney, John R.; Gramajo, Hugo (2006). "In vivo characterization of the dTDP-D-desosamine pathway of the megalomicin gene cluster from Micromonospora megalomicea". Microbiology. 152 (3): 667–673. doi:10.1099/mic.0.28680-0. PMID 16514147.
- Flickinger, Michael C.; Perlman, D. (1975). "Microbial degradation of erythromycins A and B". The Journal of Antibiotics. 28 (4): 307–311. doi:10.7164/antibiotics.28.307. PMID 1150530.
- Burgie, E. Sethe; Holden, Hazel M. (2007). "Molecular architecture of DesI: A key enzyme in the biosynthesis of desosamine". Biochemistry. 46 (31): 8999–9006. doi:10.1021/bi700751d. PMC 2528198.
- Bolton, C. H.; Foster, A. B.; Stacey, M.; Webber, J. M. (1961). "Carbohydrate components of antibiotics. Part I. Degradation of desosamine by alkali: Its absolute configuration at position 5". Journal of the Chemical Society. 1961 (4): 4831–4836. doi:10.1039/JR9610004831.
- Berry, Martyn (1963). "The macrolide antibiotics". Quarterly Reviews, Chemical Society. 17 (4): 343–361. doi:10.1039/QR9631700343.
