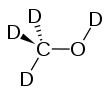
Deuterated methanol
Deuterated methanol (CD3OD), is a form (called an isotopologue) of methanol (CH3OH) in which the hydrogen atom ("H") is replaced with deuterium (heavy hydrogen) isotope ("D"). Deuterated methanol is a common solvent used in NMR spectroscopy.
| |||
| Identifiers | |||
|---|---|---|---|
3D model (JSmol) |
|||
| 1733278 | |||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.011.253 | ||
| EC Number |
| ||
PubChem CID |
|||
| UN number | 1230 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| CD4O | |||
| Molar mass | 36.0665 g mol−1 | ||
| Density | 0.888 g cm−3 | ||
| Melting point | −98 °C (−144 °F; 175 K) | ||
| Boiling point | 65 °C (149 °F; 338 K) | ||
| Thermochemistry | |||
Heat capacity (C) |
87.9 J K−1 mol−1 | ||
| Hazards | |||
| GHS pictograms |    | ||
| GHS Signal word | Warning | ||
| H225, H301, H311, H331, H370 | |||
| P210, P233, P240, P241, P242, P243, P260, P261, P264, P270, P271, P280, P301+310, P302+352, P303+361+353, P304+340, P307+311, P311, P312, P321, P322, P330, P361, P363, P370+378 | |||
| Flash point | 11 °C (52 °F; 284 K) | ||
| Related compounds | |||
Related compounds |
Methanol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.