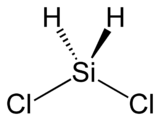
Dichlorosilane
Dichlorosilane, or DCS as it is commonly known, is a chemical compound with the formula H2SiCl2. In its major use, it is mixed with ammonia (NH3) in LPCVD chambers to grow silicon nitride in semiconductor processing. A higher concentration of DCS·NH3 (i.e. 16:1), usually results in lower stress nitride films.
 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Dichlorosilane[1] | |||
| Other names
Silicic dichloride dihydride | |||
| Identifiers | |||
3D model (JSmol) |
|||
| Abbreviations | DCS | ||
| ChemSpider | |||
| ECHA InfoCard | 100.021.717 | ||
| EC Number |
| ||
| MeSH | dichlorosilane | ||
PubChem CID |
|||
| RTECS number |
| ||
| UN number | 2189 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| SiH 2Cl 2 | |||
| Molar mass | 101.007 g mol−1 | ||
| Appearance | Colourless gas | ||
| Density | 4.228 g cm−3 | ||
| Melting point | −122 °C (−188 °F; 151 K) | ||
| Boiling point | 8 °C; 46 °F; 281 K at 101 kPa | ||
| Reacts | |||
| Vapor pressure | 167.2 kPa (at 20 °C) | ||
| Thermochemistry | |||
Std molar entropy (S |
286.72 J K−1 mol−1 | ||
Std enthalpy of formation (ΔfH⦵298) |
−320.49 kJ mol−1 | ||
| Hazards | |||
| Safety data sheet | inchem.org | ||
| GHS pictograms |    | ||
| GHS Signal word | Danger | ||
| H220, H250, H280, H314, H330 | |||
| P210, P261, P305+351+338, P310, P410+403 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −37 °C (−35 °F; 236 K) | ||
| 55 °C (131 °F; 328 K)[2] | |||
| Explosive limits | 4.1–99% | ||
| Related compounds | |||
Related dichlorosilanes |
Trichlorosilane | ||
Related compounds |
Dichloromethane | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
History
Dichlorosilane was originally prepared in 1919 by the gas-phase reaction of monosilane, SiH4, with hydrogen chloride, HCl, and then reported by Stock and Somieski.[3] It was found that in the gas phase, dichlorosilane will react with water vapor to give a gaseous monomeric prosiloxane, H2SiO. Prosiloxane polymerizes rapidly in the liquid phase and slowly in the gas phase, which results in liquid and solid polysiloxanes [H2SiO]n. The liquid portion of the product, which is collected via vacuum distillation, becomes viscous and gelled at room temperature. Hydrolysis was done on a solution of H2SiCl2 in benzene by brief contact with water, and the molecular weight was determined to be consistent with an average composition of [H2SiO]6. Through analytical and molecular weight determinations, n was decided to be between 6 and 7. Then, through more experimentation with the product, it was determined that n increases as time increases. After being in contact with the aqueous hydrolysis medium for a longer period of time, a polymer, [HSi(OH)O]n, was produced. There was limited availability of dichlorosilane until the silicone industry grew.
Reactions and formation
Most dichlorosilane results as a byproduct of the reaction of HCl with silicon, a reaction intended to give trichlorosilane.
Disproportionation of trichlorosilane is the preferred route.[4]
- 2 SiHCl3 ⇌ SiCl4 + SiH2Cl2
Hydrolysis
Stock and Somieski completed the hydrolysis of dichlorosilane by putting the solution of H2SiCl2 in benzene in brief contact with a large excess of water.[3][5] A large-scale hydrolysis was done in a mixed ether/alkane solvent system at 0 °C, which gave a mixture of volatile and nonvolatile [H2SiO]n. Fischer and Kiegsmann attempted the hydrolysis of dichlorosilane in hexane, using NiCl2⋅6H2O as the water source, but the system failed.[3] They did, however, complete the hydrolysis using dilute Et2O/CCl4 at -10 °C. The purpose of completing the hydrolysis of dichlorosilane is to collect the concentrated hydrolysis products, distill the solution, and retrieve a solution of [H2SiO]n oligomers in dichloromethane.[3] These methods were used to obtain cyclic polysiloxanes.
Another purpose for hydrolyzing dichlorosilane is to obtain linear polysiloxanes, and can be done by many different complex methods.[5] The hydrolysis of dichlorosilane in diethyl ether, dichloromethane, or pentane gives cyclic and linear polysiloxanes.[5]
Decomposition
Su and Schlegal studied the decomposition of dichlorosilane using transition state theory (TST) using calculations at the G2 level. Wittbrodt and Schlegel worked with these calculations and improved them using the QCISD(T) method.[6] The primary decomposition products were determined by this method to be SiCl2 and SiClH.[6]
Ultrapurification
Dichlorosilane must be ultrapurified and concentrated in order to be used for the manufacturing of semiconducting[4] epitaxial silicon layers, which are used for microelectronics. The buildup of the silicon layers produces thick epitaxial layers, which creates a strong structure.[4]
Advantage of use
Dichlorosilane is used as a starting material for semiconducting silicon layers found in microelectronics. It is used because it decomposes at a lower temperature and has a higher growth rate of silicon crystals.[4]
Safety hazards
It is a chemically active gas, which will readily hydrolyze and self ignite in air. Dichlorosilane is also very toxic, and preventative measures must be used for any experiment involving the use of the chemical.[7] Safety hazards also includes skin and eye irritation and inhalation.[8]
References
- "nchem.403-comp13 - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 27 March 2005. Identifiers and Related Records. Retrieved 30 November 2011.
- http://encyclopedia.airliquide.com/Encyclopedia.asp?GasID=23
- Seyferth, D., Prud’Homme, C., Wiseman, G., Cyclic Polysiloxanes from the Hydrolysis of Dichlorosilane, Inorganic Chemistry, 22, 2163-2167
- Vorotyntsev, V., Mochalov, G., Kolotilova, M., Kinetics of Dichlorosilane Separation from a Mixture of Chlorosilanes by Distillation Using a Regular Packing, Theoretical Foundations of Chemical Engineering, 38(4), 355-359
- Seyferth D., Prud’Homme C., Linear Polysiloxanes from Dichlorosilane, Inorganic Chemistry, 23, 4412-4417
- Walch, S., Dateo, C., Thermal Decomposition Pathways and Rates for Silane, Chlorosilane, Dichlorosilance, and Trichlorosilane, Journal of Physical Chemistry, 105, 2015-2022
- Vorotyntsev, V., Mochalov, G., Kolotilova, Volkova, E., Gas-Chromatographic and Mass-Spectrometric Determination of Impurity Hydrocarbons in Organochlorine Compounds and Dichlorosilane, Journal of Analytical Chemistry, 61(9), 883-888
- Praxair Material Safety Data Sheet (2007)
External links
- Safety data sheet for dichlorosilane from Praxair®