Didemnin
Didemnins are cyclic depsipeptide compounds isolated from a tunicate (ascidian, or sea-squirt) of the genus Trididemnum (family of Didemnidæ) that were collected in the Caribbean Sea. They were first isolated in 1978 at the University of Illinois.[1]
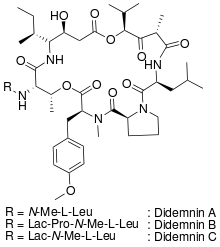
Although more than nine didemnins (didemnins A-E, G, X and Y) have been isolated from the extract of Trididemnum solidum, didemnin B is the one that possesses the most potent biological activities.[2] It is a strong antiviral agent against both DNA and RNA viruses such as herpes simplex virus type 1, a strong immunosuppressant that shows some potential in skin graft[3] and is also very cytotoxic. It shows strong activity against murine leukemia cells.[4] Large amounts of didemnin B were chemically synthesized and it was advanced to clinical trials by the National Cancer Institute. It has completed phase II human clinical trials against adenocarcinoma of the kidney,[5] advanced epithelial ovarian cancer,[6] and metastatic breast cancer.[7] Unfortunately, the compound exhibited high toxicity through a high incidence of anaphylactic reactions in patients and trials were terminated.[8]
The didemnin analog plitidepsin was in phase II clinical trials as of 2003.[9]
See also
References
- Rinehart L., K. et al.. J. Am. Chem. Soc. 1981, 103, 1857-1859.
- Rinehart L., K. et al.. J. Nat. Prod. 1988, 51, 1-21.
- Montgomery, D.; Zukoshi, C. F. Transplantation 1985, 40, 49.
- Belof, J (2006). "Survey of the didemnins: A class of depsipeptide natural products with promising biomedical applications". arXiv:q-bio/0612040.
- Taylor, S. A.; Goodman, P.; Stuckey, W. J. Stephens, R. L.; Gaynor, E. R. Invest. New Drugs 1992, 10, 55.
- Cain, J. M.; Liu, P. Y.; Alberta, D.E.; Gallion, J.J.; Laufman, L.; O'Sullivan, J.; Weiss, G.; Bickers, J. N. Invest. New Drugs 1992, 10, 113.
- Montgomery, D.; Zukoshi, C. F. Transplantation 1985, 40, 49.
- Nuijen, B.; Bouma, M.; Manada, C.; Jimeno, J.M.; Schellens, J. H. M.; Bult, A.; Beijnen, J. H. Anti-Cancer Drugs 2000, 11, 793.
- Cárdenas, F. et al. The Journal of Organic Chemistry 2003, 68 (25), 9554-9562.