Dimethyldithiocarbamate
Dimethyldithiocarbamate is the organosulfur anion with the formula (CH3)2NCS2−. It is one of the simplest organic dithiocarbamate.
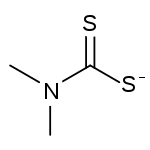
Chemical structure of the dimethyldithiocarbamate anion
Uses
It is a component of various pesticides and rubber chemicals in the form of its salts sodium dimethyldithiocarbamate, and potassium dimethyldithiocarbamate) as well as its complexes zinc dimethyldithiocarbamate, ferric dimethyldithiocarbamate, and nickel bis(dimethyldithiocarbamate). Oxidation gives thiram.[1][2]
3tight.png.webp)
Iron tris(dimethyldithiocarbamate) (Fe(S2CNMe2)3) is illustrative of hundreds of known dithiocarbamate complexes.[3]
References
- "Dimethyldithiocarbamate salts". Environmental Protection Agency. Retrieved July 26, 2016.
- Rüdiger Schubart (2000). "Dithiocarbamic Acid and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a09_001.
- D. Coucouvanis. "The Chemistry of the Dithioacid and 1,1-Dithiolate Complexes". Progress in Inorganic Chemistry. 11: 233–371. doi:10.1002/9780470166123.ch4.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.