Dinogunellin
Dinogunellins are unusual toxic phospholipids found in the roe of some fishes, and is one of the best studied ichthyotoxin.[1] These phospholipids could be found as a complex with non-toxic proteins like in the cabezon toxin or in the lipostichaerin.[2][3]
Occurrence
Dinogunellins were detected in the mature roe of four fishes: the cabezon or marbled sculpin Scorpaenichthys marmoratus, [2] the blennies Stichaeus grigorjewi [1][3] and Stichaeus nozawae, [4] and the killifish Fundulus heteroclitus. [4]
The presence of dinogunellins has been discarded in the roe of the carp Cyprinus carpio, the sculpin Hemitripterus villosus, the blenny Lumpenus fowleri,[4] and the lamprey Lapetra japonica.[5]
Structure
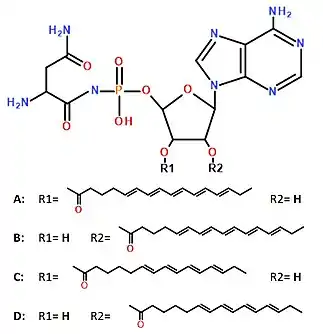
Dinogunellins are unusual phospholipids having a nucleotide instead of the typical glycerol in their structure. They consist of an adenine nucleotide, with a 2-aminosuccinimide attached to the phosphorus moiety and a fatty acid attached to the oxygen from either the C2' or the C3' of the sugar moiety.[1] The fatty acid chain could be either the eicosapentaenoic acid (Dinogunellin-A and Dinogunellin-B) or the stearidonic acid (Dinogunellin-C and Dinogunellin-D).[1] In consequence, Dinogunellin-A and Dinogunellin-B have the same molecular formula (C34H49N8O9P) and molecular weight (744.8 g/mol), an so do Dinogunellin-C and Dinogunellin-D (C32H47N8O9P; 718.7 g/mol).
Pharmacology
When intraperitoneally administered to mice, dinogunellins have a mean lethal dose (LD50) of 25 mg/kg.[3] Dinogunellins are also orally toxic to mice and guinea pigs and has also deleterious effects on humans.[6] A few hours after ingestion, humans develop abrupt onset diaphoresis, chills, abdominal pain and cramping, with nausea and vomiting followed by voluminous, non-bloody diarrhea.[7]
Analysis made on the cabezon toxin showed that its effects start 12 hours after administration and is characterized by several signs such as diarrhea, nasal discharge, and death.[8] In addition, cabezon toxin showed cytotoxicity on fibroblast in culture.[8][9] Besides, toxin administration causes an increase in white cell number, but with a decrease in lymphocytes associated with the observation of spleen necrosis. [9]
References
- Matsunaga S, Takahashi N, Fusetani N (2009-05-05). "Dinogunellins A-D: Putative ichthyootoxic phospholipids of northern blenny Stichaeus grigorjewi eggs". Pure and Applied Chemistry. 81 (6): 1001–1008. doi:10.1351/PAC-CON-08-08-28. ISSN 1365-3075. S2CID 59152160.
- Hashimoto Y, Kawasaki M, Hatano M (1976). "Occurrence of a toxic phospholipid in cabezon roe". Toxicon. 14 (2): 141–3. doi:10.1016/0041-0101(76)90105-7. PMID 1273860.
- Hatano M, Hashimoto Y (May 1974). "Properties of a toxic phospholipid in the northern blenny roe". Toxicon. 12 (3): 231–6. doi:10.1016/0041-0101(74)90063-4. PMID 4458104.
- Kamiya H, Hatano M, and Hashimoto Y. 1997. Screening of Icthyootoxin. Bulletin of the Japanese Society of Scientific Fisheries. 43(12):1461-1465.
- Shiomi K, Miyauchi K, Shimakura K, Nagashima Y (1997). "Purification and Properties of a Proteinaceous Toxin Newly Found in the Roe of Lamprey Lampetra japonica". Fisheries Science. 63 (1): 142–146. doi:10.2331/fishsci.63.142.
- Hubbs CL, Wick AN (1951). "Toxicity of the roe of the cabezon, Scorpaenichthys marmoratus". Calif Fish Game. 37: 195.
- O'Connell CW, Clark R, Villano JH, Gugelmann H, Dyer JE (August 2014). "Acute human toxicity after the ingestion of cabezon, Scorpaenichthys marmoratus, roe". Clinical Toxicology. 52 (7): 820. doi:10.3109/15563650.2014.933232. PMID 25089726.
- Fuhrman FA, Fuhrman GJ, Dull DL, Mosher HS (May 1969). "Toxins from eggs of fishes and Amphibia". Journal of Agricultural and Food Chemistry. 17 (3): 417–424. doi:10.1021/jf60163a043.
- Fuhrman FA, Fuhrman GJ, Roseen JS (May 1970). "Toxic effects produced by extracts of eggs of the cabezon Scorpaenichthys marmoratus". Toxicon. 8 (1): 55–61. doi:10.1016/0041-0101(70)90174-1. PMID 5465747.