Dioxalin
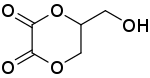
Dioxalin is a reaction product of glycerol with oxalic acid at 533 K. Its IUPAC name is 5-(hydroxymethyl)-1,4-dioxane-2,3-dione. Dioxalin readily loses two molecules of carbon dioxide at this high temperature to form allyl alcohol and therefore offers a method for conversion of glycerol to allyl alcohol.[1][2][3]
 | |
| Names | |
|---|---|
| IUPAC name
5-(Hydroxymethyl)-1,4-dioxane-2,3-dione | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
| |
| |
| Properties | |
| C5H6O5 | |
| Molar mass | 146.098 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- Chattaway, Frederick Daniel (1915). "XLVII.—The preparation of allyl alcohol". J. Chem. Soc., Trans. 107: 407–410. doi:10.1039/CT9150700407.
- Coffey, Samuel; Ward, Charles Frederick (1921). "CXLVIII.—The preparation of some allyl compounds". J. Chem. Soc., Trans. 119: 1301–1306. doi:10.1039/CT9211901301.
- Arora, Amit. Carbohydrates and Proteins. Discovery Publishing House. p. 48. ISBN 978-81-8356-178-5.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
