Dithionite
The dithionite anion ([S2O4]2−), is an oxoanion of sulfur.[1] It is commonly encountered as the salt sodium dithionite.[2]
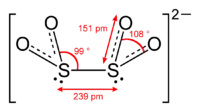
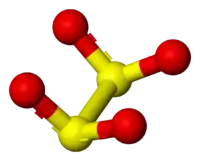
Reactions
Dithionite is a reducing agent. At pH 7, the potential is −0.66 V vs NHE. Redox occurs with formation of sulfite:[3]
- S
2O2−
4 + 2 H2O → 2 HSO−
3 + 2 e− + 2 H+
Dithionite undergoes acid hydrolytic disproportionation to thiosulfate and bisulfite:[2]
- 2 S
2O2−
4 + H2O → S
2O2−
3 + 2 HSO−
3
It also undergoes alkaline hydrolytic disproportionation to sulfite and sulfide:[2]
- 3 Na2S2O4 + 6 NaOH → 5 Na2SO3 + Na2S + 3 H2O
It is formally derived from dithionous acid (H2S2O4), but this acid does not exist in any practical sense.
Use and occurrence
Sodium dithionite finds widespread use in industry as a reducing agent.
Dithionite is used in conjunction with complexing agent (for example, citric acid) to reduce iron(III) oxy-hydroxide into soluble iron(II) compounds and to remove amorphous iron(III)-bearing mineral phases in soil analyses (selective extraction).
The decomposition of dithionite produces reduced species of sulfur that can be very aggressive for the corrosion of steel and stainless steel. Thiosulfate (S
2O2−
3) is known to induce pitting corrosion, whereas sulfide (S2−, HS−) is responsible for stress corrosion cracking (SCC).
References
- International Union of Pure and Applied Chemistry (2005). Nomenclature of Inorganic Chemistry (IUPAC Recommendations 2005). Cambridge (UK): RSC–IUPAC. ISBN 0-85404-438-8. p. 130. Electronic version.
- José Jiménez Barberá; Adolf Metzger; Manfred Wolf (15 June 2000). "Sulfites, Thiosulfates, and Dithionites". Ullmann's Encyclopedia of Industrial Chemistry. Wiley Online Library. doi:10.1002/14356007.a25_477. ISBN 978-3527306732.
- Mayhew, S. G. (2008). "The Redox Potential of Dithionite and SO2− from Equilibrium Reactions with Flavodoxins, Methyl Viologen and Hydrogen plus Hydrogenase". European Journal of Biochemistry. 85: 535–547. doi:10.1111/j.1432-1033.1978.tb12269.x.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
| Wikimedia Commons has media related to Dithionites. |
