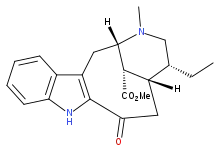
Dregamine
Dregamine is a naturally occurring monoterpene indole alkaloid found in several species in the genus Tabernaemontana including Ervatamia hirta and Tabernaemontana divaricata.
 | |
| Names | |
|---|---|
| IUPAC name
Methyl 15-ethyl-17-methyl-12-oxo-10,17-diazatetracyclo[12.3.1.03,11.04,9]octadeca-3(11),4,6,8-tetraene-18-carboxylate | |
| Preferred IUPAC name
Methyl (20α)-3-oxo-19,20-dihydrovobasan-17-oate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C21H26N2O3 | |
| Molar mass | 354.450 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
History
Dregamine was first reported in 1959 after its isolation from the apocynaceae Voacanga dregei (wild frangipani), a native small tree of southern Africa.[1] The indole alkaloid tabernaemontanine is a closely related structure, differing only in the configuration of the ethyl group in the piperidine ring.[2] Both structures are reduced versions of vobasine. There was confusion in the original literature regarding the configuration of the ethyl group in these molecules, so that their identities had been reversed.[3][4]
Synthesis
Biosynthesis
As with other Indole alkaloids, the biosynthesis of dregamine starts from the amino acid tryptophan. This is converted into strictosidine before further elaboration.[5]
Chemical synthesis
Tryptophan was used as the starting material for a synthesis of dregamine. Its single chiral center provided the correct absolute stereochemistry required when elaborated to prepare the full multi-ring system of the target product.[6]
Natural occurrence

Dregamine is found in plants of the genera Voacanga (e.g. Voacanga dregei[1]) and Tabernaemontana including Ervatamia hirta,[7] Ervatamia malaccensis[8] and Tabernaemontana divaricata.[9] The latter species is known to produce many other alkaloids including catharanthine, ibogamine and voacristine.[10]
Research
Plant metabolites have long been studied for their biological activity and alkaloids in particular are major subjects for ethnobotanical research.[11] Dragamine has been studied, for example as a potential anti-cancer agent,[12][13] for its antimalarial activity[14] and in antifertility research.[15] However, the alkaloid itself has not been developed as a drug and is known to be cardiotoxic.[16]
References
- Neuss, N.; Cone, Nancy J. (1959). "Alkaloids of apocynaceae IV. Dregamine, a new alkaloid from Voacanga dregei e. M.". Experientia. 15 (11): 414–415. doi:10.1007/BF02157683. PMID 14426722. S2CID 36370634.
- Renner, U.; Prins, D. A. (1961). "Voacanga-Alkaloide V. Verknüpfung von Vobasin mit Dregamin und Tabernaemontanin". Experientia. 17 (5): 209. doi:10.1007/BF02160617. PMID 13740864. S2CID 35816536.
- Knox, JR; Slobbe, J. (1975). "Indole alkaloids from Ervatamia orientalis. III. The configurations of the ethyl side chains of dregamine and tabernaemontanine and some further chemistry of the vobasine group". Australian Journal of Chemistry. 28 (8): 1843. doi:10.1071/CH9751843.
- Bombardelli, Ezio; Bonati, Attilio; Gabetta, Bruno; Martinelli, Ernesto M.; Mustich, Giuseppe; Danieli, Bruno (1976). "Structures of tabernaelegantines A–D and tabernaelegantinines a and B, new indole alkaloids from Tabernaemontana elegans". Journal of the Chemical Society, Perkin Transactions 1 (13): 1432–1438. doi:10.1039/P19760001432.
- Dewick, Paul M (2002). Medicinal Natural Products. A Biosynthetic Approach. Second Edition. Wiley. pp. 350–359. ISBN 0-471-49640-5.
- Kutney, James P.; Eigendorf, Gunter K.; Matsue, Hajime; Murai, Akio; Tanaka, Kiyoshi; Sung, Wing L.; Wada, Kojiro; Worth, Brian R. (1978). "Total synthesis of dregamine and epidregamine. A general route to 2-acylindole alkaloids". Journal of the American Chemical Society. 100 (3): 938–943. doi:10.1021/ja00471a047.
- Clivio, Pascale; Richard, Bernard; Deverre, Jean-Robert; Sevenet, Thierry; Zeches, Monique; Le Men-Oliver, Louisette (January 1991). "Alkaloids from leaves and root bark of Ervatamia hirta". Phytochemistry. 30 (11): 3785–3792. doi:10.1016/0031-9422(91)80111-D.
- Clivio, Pascale; Richard, Bernard; Zeches, Monique; Le Men-Olivier, Louisette; Goh, Swee Hock; David, Bruno; Sevenet, Thierry (1990). "Alkaloids from the leaves and stem bark of Ervatamia malaccensis". Phytochemistry. 29 (8): 2693–2696. doi:10.1016/0031-9422(90)85216-3.
- Kam, Toh-Seok; Pang, Huey-Shen; Lim, Tuck-Meng (2003). "Biologically active indole and bisindole alkaloids from Tabernaemontana divaricata". Organic & Biomolecular Chemistry. 1 (8): 1292–1297. doi:10.1039/B301167D. PMID 12929658.
- Kulshreshtha, Ankita; Saxena, Jyoti (2019). "Alkaloids and Non Alkaloids of Tabernaemontana divaricata" (PDF). International Journal of Research and Review. 6 (8): 517–524.
- Babiaka, Smith B.; Ntie-Kang, Fidele; Lifongo, Lydia L.; Ndingkokhar, Bakoh; Mbah, James A.; Yong, Joseph N. (2015). "The chemistry and bioactivity of Southern African flora I: A bioactivity versus ethnobotanical survey of alkaloid and terpenoid classes". RSC Advances. 5 (54): 43242–43267. doi:10.1039/C5RA01912E.
- Paterna, Angela; Kincses, Annamária; Spengler, Gabriella; Mulhovo, Silva; Molnár, Joseph; Ferreira, Maria-José U. (2017). "Dregamine and tabernaemontanine derivatives as ABCB1 modulators on resistant cancer cells". European Journal of Medicinal Chemistry. 128: 247–257. doi:10.1016/j.ejmech.2017.01.044. PMID 28189906.
- Ferreira, Maria-José U.; Paterna, Angela (2019). "Monoterpene indole alkaloids as leads for targeting multidrug resistant cancer cells from the African medicinal plant Tabernaemontana elegans". Phytochemistry Reviews. 18 (4): 971–987. doi:10.1007/s11101-019-09615-1. S2CID 184483520.
- Bapela, M. Johanna; Heyman, Heino; Senejoux, Francois; Meyer, J.J. Marion (2019). "1H NMR-based metabolomics of antimalarial plant species traditionally used by Vha-Venda people in Limpopo Province, South Africa and isolation of antiplasmodial compounds". Journal of Ethnopharmacology. 228: 148–155. doi:10.1016/j.jep.2018.07.022. OSTI 1463337. PMID 30048730.
- Jain, Sachin; Jain, Avijeet; Deb, Lokesh; Dutt, K.R.; Jain, Deepak Kumar (2010). "Evaluation of anti-fertility activity of Tabernaemontana divaricata(Linn) R.Br. Leaves in rats". Natural Product Research. 24 (9): 855–860. doi:10.1080/14786410903314385. PMID 20306358. S2CID 41422612.
- E. Burrows, George; J. Tyrl, Ronald (29 January 2013). Toxic Plants of North America. pp. 117–118. ISBN 978-0813820347.
Further reading
- Kitajima, Mariko; Takayama, Hiromitsu (2016). Monoterpenoid Bisindole Alkaloids. The Alkaloids: Chemistry and Biology. 76. pp. 259–310. doi:10.1016/bs.alkal.2015.09.001. ISBN 9780128046821. PMID 26827885.