Ethylmagnesium bromide
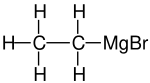
Ethylmagnesium bromide is a Grignard reagent with formula C2H5MgBr. It is widely used in the laboratory synthesis of organic compounds.
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.011.935 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C2H5BrMg | |
| Molar mass | 133.271 g·mol−1 |
| Hazards | |
| Safety data sheet | Oxford MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Reactions
Apart from acting as the synthetic equivalent of an ethyl anion synthon for nucleophilic addition, ethylmagnesium bromide may be used as a strong base to deprotonate various substrates such as alkynes:[1][2][3]
- RC≡CH + EtMgBr → RC≡CMgBr + EtH
In this application, ethylmagnesium bromide has been supplanted by the wide availability of organolithium reagents.
Preparation
Ethylmagnesium bromide is commercially available, usually as a solution in diethyl ether or tetrahydrofuran. It may be prepared in the normal manner of Grignard reagents — by reacting bromoethane with magnesium in diethyl ether:[4]
- EtBr + Mg → EtMgBr
References
- Taniguchi, H.; Mathai, I. M.; Miller, S. I. (1970). "1-Phenyl-1,4-Pentadiyne and 1-Phenyl-1,3-Pentadiyne". Organic Syntheses. 50: 97.; Collective Volume, 6, p. 925
- Quillinan, A. J.; Scheinmann, F. (1978). "3-Alkyl-1-alkynes Synthesis: 3-Ethyle-1-hexyne". Organic Syntheses. 58: 1.; Collective Volume, 6, p. 595
- Newman, M. S.; Stalick, W. M. (1977). "1-Ethoxy-1-butyne". Organic Syntheses. 57: 65.; Collective Volume, 6, p. 564
- Moyer, W. W.; Marvel, C. S. (1931). "Triethyl Carbinol". Organic Syntheses. 11: 98.; Collective Volume, 2, p. 602
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
