Evolutionary rescue
Evolutionary rescue is a theoretical situation in which a population recovers from environmental pressure through advantageous genetic change rather than increased gene flow, migration, dispersal or other demographic rescue techniques. While the term was first used in 1995 in Richard Gomulkiewicz and Robert Holt's essay in the journal Evolution,[1] the theory has since academically matured through review and modeling. The most commonly used meaning of the term was established in Gonzalez et al. (2012), which states that evolutionary rescue "occurs when genetic adaptation allows a population to recover from demographic effects initiated by environmental change that would otherwise cause extirpation".[2]
Theoretical framework
Due to rapid losses of biodiversity, climate change, and rapid spread of non-native species due to globalization, natural environments are changing faster now than in any other time point in human history. Given that traditional framework of evolution emphasizes that ecologically relevant evolution occurs slowly for the plant and animal kingdoms, the 20th century and 21st century shift in the rate of environmental change naturally raises questions of contemporary evolution. The central question in the framework of evolutionary rescue remains, "If contemporary evolution, also referred to as rapid evolution, occurs, can it be fast enough to foster population persistence in extremely small time-scales?". Proponents of the evolutionary rescue hypothesis argue that evolutionary rescue is a unique phenomenon, separate from two commonly studied escapes from ecological pressures, demographic and genetic rescues. While dispersal is a key aspect to all three population survival strategies,[3] evolutionary rescue is the only one driven by adaptive evolution mechanistically.[4] Demographic rescue is simply a dynamic driven by source-sink immigration dynamics, whereas genetic rescue is primarily driven by hybridization in combination with immigration. Given the reliance on immigration, it can be difficult to disentangle demographic rescue from genetic rescue. The distinction between these three rescue strategies may be difficult to prove, if it exists, although rescue from negative population growth may be a hallmark.
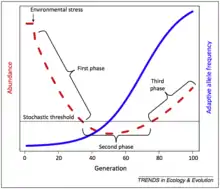
It may be easier to find quantifiable evidence for evolutionary rescue in rapidly shifting environments versus gradually shifting environments (climate change, ocean acidification, etc.). High localization and source-sink biogeographic demographic dynamics may also increase the likelihood of evolutionary rescue. Evolutionary rescue may also be highly characterized by a U-shaped curve, in which the population declines sharply until it passes a stochastic threshold, at which the adaptive allele frequency rapidly increases, with the population rebounding at a slower rate as well.[5] The most vulnerable part of the evolutionary rescue process, theoretically, should be the time point during which the population is beyond the stochastic threshold, which exposes the population to random outcomes not determined by genetic or evolutionary mechanisms.
Empirical evidence
While there is empirical evidence for evolutionary rescue, it is important to note that missing temporal, demographic or allelic data makes the majority of these examples suggestive of evolutionary rescue at best. For example, the demographic shifts in Gasterosteus aculeatus from weakly armored to heavily armored variants following bioremediation of water clarity in Lake Washington, USA, suggest that increased the predatory efficiency of Oncorhynchus clarkii,[6] although inclusion versus exclusion treatments at controlled densities would provide stronger mechanistic support. Another possible example of evolutionary rescue in nature is the selective response of redbellied black snakes (Pseudechis porphyriacus) to cane toads (Rhinella marina) in Australia, in which P. porphyriacus exposed to R. marina for long periods of time demonstrated rapid adaptive shifts.[7] Finally, some little brown bats (Myotis lucifugus) affected by the exotic disease white-nose syndrome (caused by the introduced pathogen Pseudogymnoascus destructans) are genetically less susceptible, but only time will tell if the adaptations are beneficial enough to allow populations to recover in the presence of the disease.[8]
Unresolved questions
At this point in time, more substantial theoretical, modeling and empirical work is needed to distinguish evolutionary rescue from traditionally adaptation discourse in the evolutionary biology field. Currently, one of the key arguments separating evolutionary rescue from adaptation is the reliance on absolute fitness versus relative fitness.[9] While the theory of evolutionary rescue may help inform future conservation efforts, more work is needed to explain the role of the interplay between dispersal and negative density dependence on an organism's short-term and long-term capacity for adaptation and evolutionary rescue. Furthermore, more in-depth work on the role of stochastic fluctuations, particularly in the determination of stochastic thresholds, is needed to establish evolutionary rescue as a viable explanation for trends in evolutionary biology.
See also
- Genetic rescue, a man-made edition of this process
References
- Gomulkiewicz, Richard; Holt, Robert (1995). "When does natural selection save a population from extinction?". Evolution. 49 (1): 201–207. doi:10.1111/j.1558-5646.1995.tb05971.x. PMID 28593677. S2CID 29819056.
- Gonzalez, Andrew (February 2012). "Evolutionary rescue; an emerging focus at the intersection between ecology and evolution". Philosophical Transactions of the Royal Society.
- Bell, G (2013). "Evolutionary rescue and the limits of adaptation". Philosophical Transactions of the Royal Society B. 368 (1610). doi:10.1098/rstb.2012.0080. PMC 3538447. PMID 23209162.
- Gonzales, A. (2013). "Evolutionary rescue: an emerging focus at the intersection between ecology and evolution". Philos. Trans. R. Soc. Lond. B Biol. Sci. 368 (1610). doi:10.1098/rstb.2012.0404. PMC 3538460. PMID 23209175.
- Gomulkiewicz, R. (1995). "When does evolution by natural selection prevent extinction?". Evolution. 49 (1): 201–207. doi:10.1111/j.1558-5646.1995.tb05971.x. PMID 28593677. S2CID 29819056.
- Kitano, J (2008). "Reverse evolution of armor plates in the threespine stickleback" (PDF). Current Biology. 18 (10): 769–774. doi:10.1016/j.cub.2008.04.027. PMID 18485710. S2CID 7864384.
- Phillips, B.L; Shine, R (2006). "An invasive species induces rapid adaptive change in a native predator: cane toads and black snakes in Australia". Proceedings: Biological Sciences. 273 (1593): 1545–50. doi:10.1098/rspb.2006.3479. PMC 1560325. PMID 16777750.
- Auteri, Giorgia G.; Knowles, Lacey L. (2020). "Decimated little brown bats show potential for adaptive change". Scientific Reports. 10 (1): 3023. Bibcode:2020NatSR..10.3023A. doi:10.1038/s41598-020-59797-4. PMC 7033193. PMID 32080246.
- Kliman, Richard (2016). AN Encyclopedia of Evolutionary Biology. Academic Press.
