Exothermic reaction
An exothermic reaction is a "reaction for which the overall standard enthalpy change ΔH⚬ is negative."[1][2] Exothermic reactions usually release heat and entail the replacement of weak bonds with stronger ones.[3][4] The term is often confused with exergonic reaction, which IUPAC defines as "... a reaction for which the overall standard Gibbs energy change ΔG⚬ is negative."[2] A strongly exothermic reaction will usually also be exergonic because ΔH⚬ makes a major contribution to ΔG⚬. Most of the spectacular chemical reactions that are demonstrated in classrooms are exothermic and exergonic. The opposite is an endothermic reaction, which usually takes up heat and is driven by an entropy increase in the system.
Examples
Examples are numerous: combustion, the thermite reaction, combining strong acids and bases, polymerizations. As an example in everyday life, hand warmers make use of the oxidation of iron to achieve an exothermic reaction:
- 4Fe + 3O2 → 2Fe2O3 ΔH⚬ = - 1648 kJ/mol
A particularly important class of exothermic reactions is combustion of a hydrocarbon fuel, e.g. the burning of natural gas:
- ΔH⚬ = - 890 kJ/mol
In these examples, most of the energy released was stored in O2 with its relatively weak double bond.[4] Most chemical reactions involve both the breaking of existing and the making of new, stronger chemical bonds. When atoms come together to form the new, more stable chemical bonds, the electrostatic forces bringing them together leave the bond with a large excess of energy (usually in the form of vibrations and rotations). If that energy is not dissipated, the new bond would quickly break apart again. Instead, the new bond can shed its excess energy - by radiation, by transfer to other motions in the molecule, or to other molecules through collisions - and then become a stable new bond. This excess energy is the heat that leaves the molecular system.
Uncontrolled exothermic reactions, those leading to fires and explosions, are wasteful because it is difficult to capture the released energy. Nature effects combustion reactions under highly controlled conditions, avoiding fires and explosions, in aerobic respiration so as to capture the released energy, e.g. for the formation of ATP.
Measurement
The enthalpy of a chemical system is essentially its energy. The enthalpy change ΔH for a reaction is equal to the heat q transferred out of (or into) a closed system at constant pressure without in- or output of electrical energy. Heat production or absorption in a chemical reaction is measured using calorimetry, e.g. with a bomb calorimeter. One common laboratory instrument is the reaction calorimeter, where the heat flow from or into the reaction vessel is monitored. The heat release and corresponding energy change, ΔH, of a combustion reaction can be measured particularly accurately.
The measured heat energy released in an exothermic reaction is converted to ΔH⚬ in Joule per mole (formerly cal/mol). The standard enthalpy change ΔH⚬ is essentially the enthalpy change when the stoichiometric coefficients in the reaction are considered as the amounts of reactants and products (in mole); usually, the initial and final temperature is assumed to be 25 °C. For gas-phase reactions, ΔH⚬ values are related to bond energies to a good approximation by:
- ΔH⚬ = total bond energy of reactants − total bond energy of products
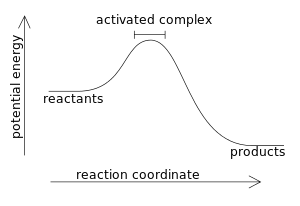
In an exothermic reaction, by definition, the enthalpy change has a negative value:
- ΔH = Hproducts - Hreactants < 0
where a larger value (the higher energy of the reactants) is subtracted from a smaller value (the lower energy of the products). For example, when hydrogen burns:
- 2H2 (g) + O2 (g) → 2H2O (g)
- ΔH⚬ = −483.6 kJ/mol [5]
See also
References
- "Exothermic reaction". IUPAC.
- Laidler, K. J. (1996). "A glossary of terms used in chemical kinetics, including reaction dynamics (IUPAC Recommendations 1996)". Pure and Applied Chemistry. 68: 149–192. doi:10.1351/pac199668010149. S2CID 98267946.
- Galley, William C. (2004). "Exothermic Bond Breaking: A Persistent Misconception". Journal of Chemical Education. 81 (4): 523. Bibcode:2004JChEd..81..523G. doi:10.1021/ed081p523.
- Schmidt-Rohr, Klaus (2015). "Why Combustions Are Always Exothermic, Yielding About 418 kJ per Mole of O2". Journal of Chemical Education. 92 (12): 2094–2099. Bibcode:2015JChEd..92.2094S. doi:10.1021/acs.jchemed.5b00333.
- "Archived copy". Archived from the original on 2013-07-08. Retrieved 2013-07-20.CS1 maint: archived copy as title (link)
