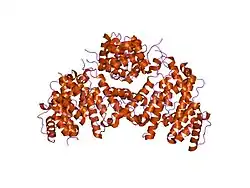
Fimbrin
Fimbrin also known as is plastin 1 is a protein that in humans is encoded by the PLS1 gene.[5] Fimbrin is an actin cross-linking protein important in the formation of filopodia.
Structure
Fimbrin belongs to the calponin homology (CH) domain superfamily of actin cross-linking proteins. Like other members of this superfamily, which include α-actinin, β-spectrin, dystrophin, ABP-120 and filamin, it has a conserved 27 kDa actin-binding domain that contains a tandem duplication of a sequence that is homologous to calponin. In addition to cross-linking actin filaments into bundles and networks, CH domains also bind intermediate filaments and some signal transduction proteins to the actin cytoskeleton. Structural comparison of actin filaments and fimbrin CH domain-decorated actin filaments has revealed changes in the actin structure due to fimbrin-mediated cross-linking that may affect the actin filaments' affinity for other actin-binding proteins and may be part of the regulation of the cytoskeleton itself.[6]
In humans, three highly homologous, strictly tissue and locale specific isoforms have been identified: I-, T- and L-fimbrin.[6] L-fimbrin is found in only normal or transformed leukocytes where it becomes phosphorylated in response to other factors such as interleukin-1. I-fimbrin is expressed by intestine and kidney epithelial cells.[7] T-fimbrin is found in epithelial and mesenchymal cells derived from solid tissue where it does not become phosphorylated. Differences in expression, sequence and phosphorylation among the various fimbrin isoforms suggest the likelihood of functional differences.[7]
Function
Fimbrin is present in several distinct structures in different cell types, including intestinal microvilli, hair cell stereocilia and fibroblast filopodia.[7] It is usually associated with polarized actin filaments in membrane ruffles, filopodia, stereocilia and adhesion plaques. Sequence homology and biochemical properties show that fimbrin is highly conserved from yeast to humans. Yeast mutants lacking fimbrin are defective in morphogenesis and endocytosis.[6]
Owing to the close proximity of its tandem actin-binding domains, fimbrin directs the formation of tightly bundled actin filaments that participate in dynamic processes, including cytokinesis in yeast and host cell invasion by enteropathic bacteria. Although fimbrin's involvement in processes like these as well as its role in assembly and regulation of microfilament networks are well documented, there are fewer experimental data describing the overall domain organization of the molecule. Klein et al. (2004) detailed the crystal structure of the Arabidopsis thaliana and Schizosaccharomyces pombe fimbrin cores in an attempt to highlight the compact and distinctly asymmetric organization of the fimbrin molecule. This structural study of the fimbrin core represents the first detailed structural description of a functional actin cross-linking protein.[8]
References
- GRCh38: Ensembl release 89: ENSG00000120756 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000049493 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Entrez Gene: Plastin 1".
- de Arruda MV, Watson S, Lin CS, Leavitt J, Matsudaira P (September 1990). "Fimbrin is a homologue of the cytoplasmic phosphoprotein plastin and has domains homologous with calmodulin and actin gelation proteins". J. Cell Biol. 111 (3): 1069–79. doi:10.1083/jcb.111.3.1069. PMC 2116281. PMID 2391360.
- Chafel MM, Shen W, Matsudaira P (Jun 1995). "Sequential expression and differential localization of I-, L-, and T-fimbrin during differentiation of the mouse intestine and yolk sac". Dev Dyn. 203 (2): 141–51. doi:10.1002/aja.1002030203. PMID 7655078. S2CID 20594198.
- Klein MG, Shi W, Ramagopal U, Tseng Y, Wirtz D, Kovar DR, Staiger CJ, Almo SC (June 2004). "Structure of the actin crosslinking core of fimbrin" (PDF). Structure. 12 (6): 999–1013. doi:10.1016/j.str.2004.04.010. PMID 15274920.
Further reading
- Hanein D, Matsudaira P, DeRosier DJ (October 1997). "Evidence for a conformational change in actin induced by fimbrin (N375) binding". J. Cell Biol. 139 (2): 387–96. doi:10.1083/jcb.139.2.387. PMC 2139807. PMID 9334343.
- Lodish H, Berk A, Zipursky L, Matsudaira P, Baltimore D, Darnell J (1999). "Section 18.1: The Actin Cytoskeleton". Molecular Cell Biology (4th ed.). New York; Houndsmills: W. H. Freeman & Co. ISBN 978-0-7167-3706-3.