Flavocytochrome c sulfide dehydrogenase
Flavocytochrome c sulfide dehydrogenase, also known as Sulfide-cytochrome-c reductase (flavocytochrome c) (EC 1.8.2.3), is an enzyme with systematic name hydrogen-sulfide:flavocytochrome c oxidoreductase.[1][2][3][4][5][6] It is found in sulfur-oxidising bacteria such as the purple phototrophic bacteria Allochromatium vinosum.[4][7] This enzyme catalyses the following chemical reaction:
- hydrogen sulfide + 2 ferricytochrome c sulfur + 2 ferrocytochrome c + 2 H+
| Sulfide-cytochrome-c reductase (flavocytochrome c) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
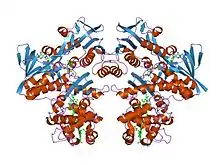 Structure of the flavocytochrome c sulfide dehydrogenase from the purple phototrophic bacterium Allochromatium vinosum (PDB: 1FCD). | |||||||||
| Identifiers | |||||||||
| EC number | 1.8.2.3 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
| Flavocytochrome c sulfide dehydrogenase, flavin-binding | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | FCSD-flav_bind | ||||||||
| Pfam | PF09242 | ||||||||
| InterPro | IPR015323 | ||||||||
| SCOP2 | 1fcd / SCOPe / SUPFAM | ||||||||
| |||||||||
These enzymes are heterodimers of a flavoprotein (fccB Q06530) and a dihaem cytochrome (fccA; Q06529) that carry out hydrogen sulfide-dependent cytochrome C reduction. The dihaem cytochrome folds into two domains, each of which resembles mitochondrial cytochrome c, with the two haem groups bound to the interior of the subunit. The flavoprotein subunit has a glutathione reductase-like fold consisting of a beta(3,4)-alpha(3) core, and an alpha+beta sandwich. The active site of the flavoprotein subunit contains a catalytically important disulfide bridge located above the pyrimidine portion of the flavin ring. The flavoprotein contains a C-terminal domain required for binding to flavin, and subsequent electron transfer.[4] Electrons are transferred from the flavin to one of the haem groups in the cytochrome. Both FAD and heme C are covalently bound to the protein.
References
- Kusai K, Yamanaka T (November 1973). "The oxidation mechanisms of thiosulphate and sulphide in Chlorobium thiosulphatophilum: roles of cytochrome c-551 and cytochrome c-553". Biochimica et Biophysica Acta (BBA) - Bioenergetics. 325 (2): 304–14. doi:10.1016/0005-2728(73)90106-0. PMID 4357558.
- Fukumori Y, Yamanaka T (June 1979). "Flavocytochrome c of Chromatium vinosum. Some enzymatic properties and subunit structure". Journal of Biochemistry. 85 (6): 1405–14. doi:10.1093/oxfordjournals.jbchem.a132467. PMID 222744.
- Gray GO, Gaul DF, Knaff DB (April 1983). "Partial purification and characterization of two soluble c-type cytochromes from Chromatium vinosum". Archives of Biochemistry and Biophysics. 222 (1): 78–86. doi:10.1016/0003-9861(83)90504-0. PMID 6301383.
- Chen ZW, Koh M, Van Driessche G, Van Beeumen JJ, Bartsch RG, Meyer TE, Cusanovich MA, Mathews FS (October 1994). "The structure of flavocytochrome c sulfide dehydrogenase from a purple phototrophic bacterium". Science. 266 (5184): 430–2. doi:10.1126/science.7939681. PMID 7939681.
- de Jong GA, Robertson LA, Kuenen GJ (May 1998). "Purification and characterization of sulfide dehydrogenase from alkaliphilic chemolithoautotrophic sulfur-oxidizing bacteria". FEBS Letters. 427 (1): 11–4. doi:10.1016/S0014-5793(98)00379-2. PMID 9613590. S2CID 2818096.
- Kostanjevecki V, Brigé A, Meyer TE, Cusanovich MA, Guisez Y, van Beeumen J (June 2000). "A membrane-bound flavocytochrome c-sulfide dehydrogenase from the purple phototrophic sulfur bacterium Ectothiorhodospira vacuolata". Journal of Bacteriology. 182 (11): 3097–103. doi:10.1128/jb.182.11.3097-3103.2000. PMC 94494. PMID 10809687.
- Quentmeier A, Hellwig P, Bardischewsky F, Wichmann R, Friedrich CG (November 2004). "Sulfide dehydrogenase activity of the monomeric flavoprotein SoxF of Paracoccus pantotrophus". Biochemistry. 43 (46): 14696–703. doi:10.1021/bi048568y. PMID 15544340.
External links
- Sulfide-cytochrome-c+reductase+(flavocytochrome+c) at the US National Library of Medicine Medical Subject Headings (MeSH)