Flosequinan
Flosequinan is a quinolone vasodilator that was discovered and developed by Boots UK and was sold for about a year under the trade name Manoplax. It had been approved in 1992 in the US and UK to treat people with heart failure who could not tolerate ACE inhibitors or digitalis.[1]
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
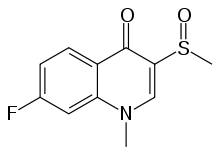
| Formula | C11H10FNO2S |
| Molar mass | 239.26 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Boots initiated a clinical trial called PROFILE to see if the drug could be useful in a wider population. The study was terminated early in 1993 due to increased mortality in the drug arm of the trial; preliminary results were published in a conference abstract by the PI Milton Packer and others, which promised data and analysis would be forthcoming in a future paper,[2] which was finally published in 2017.[3]
References
- Hosking P (25 July 1993). "Manoplax: from heart to heartbreak: With millions lost on its 'wonder". The Independent.
- van Veldhuisen DJ, Poole-Wilson PA (August 2001). "The underreporting of results and possible mechanisms of 'negative' drug trials in patients with chronic heart failure". International Journal of Cardiology. 80 (1): 19–27. doi:10.1016/s0167-5273(01)00447-8. PMID 11532543.
- Packer M, Pitt B, Rouleau JL, Swedberg K, DeMets DL, Fisher L (June 2017). "Long-Term Effects of Flosequinan on the Morbidity and Mortality of Patients With Severe Chronic Heart Failure: Primary Results of the PROFILE Trial After 24 Years". JACC. Heart Failure. 5 (6): 399–407. doi:10.1016/j.jchf.2017.03.003. PMID 28501522.
- Associated Press (July 19, 1993). "Heart Failure Drug Manoplax Taken Off Market". AP News Archive.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.