Fluorite structure
In solid state chemistry, the fluorite structure refers to a common motif for compounds with the formula MX2.[1][2] The X ions occupy the eight tetrahedral interstitial sites whereas M ions occupy the regular sites of a face-centered cubic (FCC) structure. Many compounds, notably the common mineral fluorite (CaF2), adopt this structure.
Magnesium compounds such as Mg2X, where X can be one of the elements Si, Ge, Sn, or Pb, are said to have an antifluorite structure because the locations of the anions and cations are reversed relative to fluorite (an anti-structure); the anions occupy the FCC regular sites whereas the cations occupy the tetrahedral interstitial sites. Magnesium silicide, Mg2Si, has a lattice parameter of 6.338 Å with magnesium cations occupying the tetrahedral interstitial sites, in which each silicide anion is surrounded by eight magnesium cations and each magnesium cation is surrounded by four silicide anions in a tetrahedral fashion.[3]
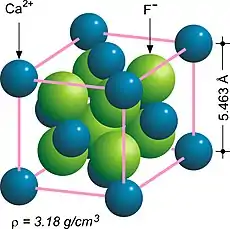 The fluorite structure of calcium fluoride CaF2.[3]
The fluorite structure of calcium fluoride CaF2.[3]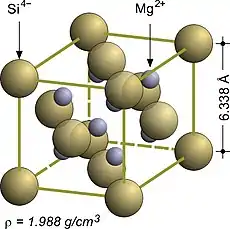 The antifluorite structure of magnesium silicide Mg2Si.[3]
The antifluorite structure of magnesium silicide Mg2Si.[3]
| Material | Lattice constant (Å) | Crystal structure | |
|---|---|---|---|
| BaF2 | 6.196 | Fluorite (FCC) | |
| β-PbF2 | 5.94 | Fluorite (FCC) | |
| PuO2 | 5.399 | Fluorite (FCC) | |
| SrF2 | 5.7996 | Fluorite (FCC) | |
| UO2 | 5.47065 | Fluorite (FCC) | |
| CaF2 | 5.463 | Fluorite (FCC) | |
| ZrO2 | 5.14 | Fluorite (FCC) | |
| K2O | 6.449 | Antifluorite (FCC) | |
| K2S | 7.406 | Antifluorite (FCC) | |
| Li2O | 4.61 | Antifluorite (FCC) | |
| Na2O | 5.55 | Antifluorite (FCC) | |
| Na2S | 6.54 | Antifluorite (FCC) | |
| Rb2O | 6.74 | Antifluorite (FCC) | |
| Mg2Si | 6.338 | Antifluorite (FCC) |
See also
References
- Holleman, Arnold Frederik; Wiberg, Egon (2001), Wiberg, Nils (ed.), Inorganic Chemistry, translated by Eagleson, Mary; Brewer, William, San Diego/Berlin: Academic Press/De Gruyter, ISBN 0-12-352651-5
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Rizescu, Costel; Rizescu, Mihaela (2018). Structure of Crystalline Solids, Imperfections and Defects in Crystals (First ed.). Parker, TX: Shutter Waves. ISBN 978-1-947641-17-4. Retrieved 29 January 2020.
