Gamma-glutamyl carboxylase
Gamma-glutamyl carboxylase is an enzyme that in humans is encoded by the GGCX gene, located on chromosome 2 at 2p12.[4]
| GGCX | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||
| Aliases | GGCX, VKCFD1, gamma-glutamyl carboxylase, Gamma-glutamyl carboxylase; GGCX | ||||||||||||||||||||||||
| External IDs | OMIM: 137167 MGI: 1927655 HomoloGene: 639 GeneCards: GGCX | ||||||||||||||||||||||||
| |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| Orthologs | |||||||||||||||||||||||||
| Species | Human | Mouse | |||||||||||||||||||||||
| Entrez | |||||||||||||||||||||||||
| Ensembl | |||||||||||||||||||||||||
| UniProt | |||||||||||||||||||||||||
| RefSeq (mRNA) | |||||||||||||||||||||||||
| RefSeq (protein) | |||||||||||||||||||||||||
| Location (UCSC) | Chr 2: 85.54 – 85.56 Mb | n/a | |||||||||||||||||||||||
| PubMed search | [2] | [3] | |||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
Function
Gamma-glutamyl carboxylase is an enzyme that catalyzes the posttranslational modification of vitamin K-dependent proteins. Many of these vitamin K-dependent proteins are involved in coagulation so the function of the encoded enzyme is essential for hemostasis.[5] Most gla domain-containing proteins depend on this carboxylation reaction for posttranslational modification.[6] In humans, the gamma-glutamyl carboxylase enzyme is most highly expressed in the liver.
Catalytic reaction
Gamma-glutamyl carboxylase oxidizes Vitamin K hydroquinone to Vitamin K 2,3 epoxide, while simultaneously adding CO2 to protein-bound glutamic acid (abbreviation = Glu) to form gamma-carboxyglutamic acid (also called gamma-carboxyglutamate, abbreviation = Gla). Presence of two carboxylate groups causes chelation of Ca2+ , resulting in change in tertiary structure of protein and its activation. The carboxylation reaction will only proceed if the carboxylase enzyme is able to oxidize vitamin K hydroquinone to vitamin K epoxide at the same time; the carboxylation and epoxidation reactions are said to be coupled reactions.[7][8][9]
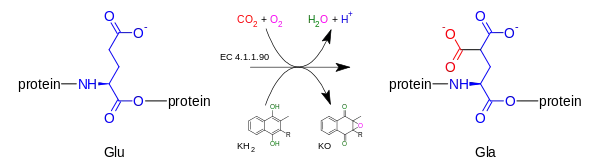
2) + CO
2 + oxygen → a [protein] 4-carboxy-L-glutamate (Gla) + vitamin K 2,3-epoxide (KO) + H+
+ H
2O
Clinical significance
Mutations in this gene are associated with vitamin K-dependent coagulation defect and PXE-like disorder with multiple coagulation factor deficiency.[5][10]
See also
References
- GRCh38: Ensembl release 89: ENSG00000115486 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Wu SM, Cheung WF, Frazier D, Stafford DW (December 1991). "Cloning and expression of the cDNA for human gamma-glutamyl carboxylase". Science. 254 (5038): 1634–6. doi:10.1126/science.1749935. PMID 1749935.
- "Entrez Gene: GGCX".
- Brenner B, Tavori S, Zivelin A, Keller CB, Suttie JW, Tatarsky I, Seligsohn U (August 1990). "Hereditary deficiency of all vitamin K-dependent procoagulants and anticoagulants". Br. J. Haematol. 75 (4): 537–42. doi:10.1111/j.1365-2141.1990.tb07795.x. PMID 2145029. S2CID 24679257.
- Suttie JW (1985). "Vitamin K-dependent carboxylase". Annu. Rev. Biochem. 54 (1): 459–77. doi:10.1146/annurev.bi.54.070185.002331. PMID 3896125.
- Presnell SR, Stafford DW (2002). "The vitamin K-dependent carboxylase". Thromb. Haemost. 87 (6): 937–46. doi:10.1055/s-0037-1613115. PMID 12083499.
- Silva PJ, Ramos MJ (2007). "Reaction mechanism of the vitamin K-dependent glutamate carboxylase: a computational study". J Phys Chem B. 111 (44): 12883–7. doi:10.1021/jp0738208. PMID 17935315.
- Vanakker OM, Martin L, Gheduzzi D, Leroy BP, Loeys BL, Guerci VI, Matthys D, Terry SF, Coucke PJ, Pasquali-Ronchetti I, De Paepe A (March 2007). "Pseudoxanthoma elasticum-like phenotype with cutis laxa and multiple coagulation factor deficiency represents a separate genetic entity". J. Invest. Dermatol. 127 (3): 581–7. doi:10.1038/sj.jid.5700610. PMID 17110937.
Further reading
- Bandyopadhyay PK (2008). "Vitamin K-dependent gamma-glutamylcarboxylation: an ancient posttranslational modification". Vitam. Horm. Vitamins & Hormones. 78: 157–84. doi:10.1016/S0083-6729(07)00008-8. ISBN 9780123741134. PMID 18374194.
- Berkner KL (2008). "Vitamin K-dependent carboxylation". Vitam. Horm. Vitamins & Hormones. 78: 131–56. doi:10.1016/S0083-6729(07)00007-6. ISBN 9780123741134. PMID 18374193.
- Oldenburg J, Marinova M, Müller-Reible C, Watzka M (2008). "The vitamin K cycle". Vitam. Horm. Vitamins & Hormones. 78: 35–62. doi:10.1016/S0083-6729(07)00003-9. ISBN 9780123741134. PMID 18374189.
- Berkner KL (2005). "The vitamin K-dependent carboxylase". Annu. Rev. Nutr. 25 (1): 127–49. doi:10.1146/annurev.nutr.25.050304.092713. PMID 16011462.
- Zhang B, Ginsburg D (September 2004). "Familial multiple coagulation factor deficiencies: new biologic insight from rare genetic bleeding disorders". J. Thromb. Haemost. 2 (9): 1564–72. doi:10.1111/j.1538-7836.2004.00857.x. hdl:2027.42/74529. PMID 15333032. S2CID 7437035.
- Wallin R, Hutson SM (July 2004). "Warfarin and the vitamin K-dependent gamma-carboxylation system". Trends Mol Med. 10 (7): 299–302. doi:10.1016/j.molmed.2004.05.003. PMID 15242675.
- Berkner KL (August 2000). "The vitamin K-dependent carboxylase". J. Nutr. 130 (8): 1877–80. doi:10.1093/jn/130.8.1877. PMID 10917896.
- Presnell SR, Stafford DW (June 2002). "The vitamin K-dependent carboxylase". Thromb. Haemost. 87 (6): 937–46. doi:10.1055/s-0037-1613115. PMID 12083499.
- Bender, David A. (2003). Nutritional biochemistry of the vitamins. Cambridge, UK: Cambridge University Press. ISBN 0-521-80388-8.
- Ball, George E. (2004). Vitamins: their role in the human body. Oxford: Blackwell Science. ISBN 0-632-06478-1.
- Combs, Gerald F. (1998). The vitamins: fundamental aspects in nutrition and health. Boston: Academic Press. ISBN 0-12-183492-1.
External links
- glutamyl+carboxylase at the US National Library of Medicine Medical Subject Headings (MeSH)
This article incorporates text from the United States National Library of Medicine, which is in the public domain.