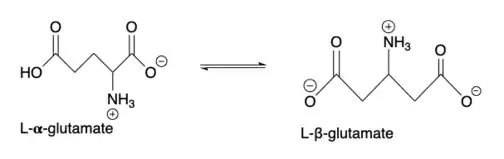
Glutamate 2,3-aminomutase
Glutamate 2,3-aminomutase (EC 5.4.3.9) is an enzyme that belongs to the radical s-adenosyl methionine (SAM) superfamily. Radical SAM enzymes facilitate the reductive cleavage of S-adenosylmethionine (SAM) through the use of radical chemistry and an iron-sulfur cluster.[1] This enzyme family is implicated in the biosynthesis of DNA precursors, vitamin, cofactor, antibiotic and herbicides and in biodegradation pathways.[1] In particular, glutamate 2,3 aminomutase is involved in the conversion of L-alpha-glutamate to L-beta-glutamate in Clostridium difficile.[2] The generalized reaction is shown below:
This enzyme is closely related to Lysine 2,3-aminomutase (LAM) and is thought to use similar cofactors and has a similar reaction mechanism. Experimental evidence suggests that glutamate 2,3 aminomutase uses a pyridoxal 5-phosphate cofactor to catalyze the reaction shown. The pyridoxal 5-phosphate cofactor (Vitamin B6) is heavily utilized by enzymes that catalyze aminoacid transformations.[3]
Proposed Mechanism
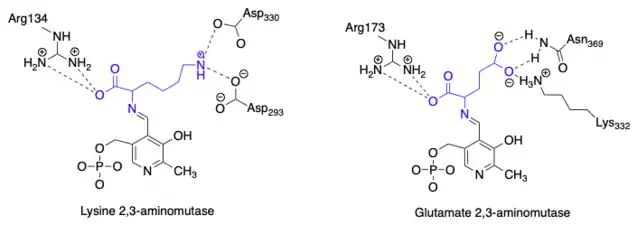
By comparing the amino acid sequences of a closely related enzyme to glutamate 2,3-aminomutase, lysine 2,3-aminomutase, researchers were able to identify key catalytic residues in glutamate 2,3-aminomutase that are distinguishing from similar aminomutases. In the case of lysine 2,3-aminomutase, lysine is bound to the enzyme in the active state, whereas glutamate 2,3-aminomutase has glutamate bound in the active state. Both enzymes appear to bind α-carboxylate groups on their respective amino acid substrates in a similar manner using arginine residues at positions 134 (lysine 2,3 aminomutase) and 173 (glutamate 2,3-aminomutase). However, the binding of the amino acid side chains differs because lysine confers basic properties where as glutamate confers acidic properties. The proposed identifying residues of a glutamate 2,3-aminomutase are Lys332 and Asn369 which likely bind the γ–carboxylate group of glutamate. This is the key difference from lysine 2,3-aminomutase because that enzyme uses Asp293 and Asp330 to bind the ε–aminium group of lysine. The proposed differences lead to a unique hydrogen bonding pattern to further distinguish glutamate 2,3-aminomutases from lysine 2,3-aminomutases which is shown here:[2]
Based on high performance liquid chromatography (HPLC) and electron paramagnetic resonance (EPR) spectroscopy techniques, the subsequent proposed glutamate 2,3-aminomutase reaction scheme is shown below:[2]
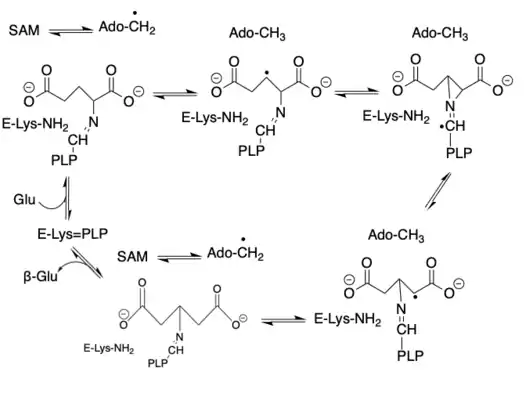
1. Starting from left-center, transaldimination occurs using L-glutamate to generate an external aldimine of PLP. This frees the active site lysine.
2. S-adenosylmethionine is reversibly cleaved to the 5'-deoxyadenosyl radical through interactions with the enzyme's iron-sulfur cluster.
3. A radical forms along with 5'-deoxyadenosyl, which remains bound to the active site.
4. Isomerization of the original radical to a second and subsequent third form of the radial occurs.
5. The third radical is β-glutamate-related and is able to abstract a hydrogen atom from 5'-deoxyadenosine.
6. The aldimine of PLP and β-glutamate undergoes another transaldimation with the free active site lysine, and releases β-glutamate and regenerates the initial PLP form to allow for another catalytic cycle.
References
- Sofia, Heidi J.; Chen, Guang; Hetzler, Beth G.; Reyes-Spindola, Jorge F.; Miller, Nancy E. (2001-03-01). "Radical SAM, a novel protein superfamily linking unresolved steps in familiar biosynthetic pathways with radical mechanisms: functional characterization using new analysis and information visualization methods". Nucleic Acids Research. 29 (5): 1097–1106. doi:10.1093/nar/29.5.1097. ISSN 0305-1048. PMC 29726. PMID 11222759.
- Ruzicka FJ, Frey PA (February 2007). "Glutamate 2,3-aminomutase: a new member of the radical SAM superfamily of enzymes". Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics. 1774 (2): 286–96. doi:10.1016/j.bbapap.2006.11.008. PMC 1945111. PMID 17222594.
- "The molecular evolution of pyridoxal-5'-phosphate-dependent enzymes". doi:10.1002/9780470123201.ch4. PMID 10800595. Cite journal requires
|journal=(help)
External links
- Glutamate+2,3-aminomutase at the US National Library of Medicine Medical Subject Headings (MeSH)