Hamiltosporidium
Hamiltosporidium is a genus of Microsporidia, which are intracellular and unicellular parasites.[1] The genus, proposed by Haag et al. in 2010, contains two species; Hamiltosporidium tvaerminnensis, and Hamiltosporidium magnivora.[2] Both species infect only the crustacean Daphnia magna (Waterflea).
| Hamiltosporidium | |
|---|---|
 | |
| Daphnia magna infected with Hamiltosporidium tvaerminnensis | |
| Scientific classification | |
| Kingdom: | |
| Division: | |
| Order: | Meiodihaplophasida |
| Family: | Duboscqiidae |
| Genus: | Hamiltosporidium Haag, 2010 |
| Species | |
|
Hamiltosporidium tvaerminnensis | |
D. magna and H. tvaerminnensis are a frequently used model organism to study coevolution and local adaptation.[3][4]
Classification
In 2010 these two species were moved to the new genus Hamiltosporidium.[2] Previously H. tvaerminnensis was wrongly identified as Octosporea bayeri Jírovec, 1936, a parasite of D. magna described in Czech Republic which was never found again. H. magnivora was formerly named Flabelliforma magnivora Larsson, 1998[5] and belonged to the Flabelliforma genus, before its close phylogenetic relation to H. tvaerminnensis was discovered.
Distribution
Infection of Daphnia populations by Hamiltosporidium have been recorded in the United Kingdom, Russia, Belgium and Israel (H. magnivora),[6] as well as Sweden, Finland and Israel (H. tvaerminnensis).[7][8] While its host D. magna is found all over the northern hemisphere, the two Hamiltosporidium species seem to have a limited geographic distribution. Genetic differences in host susceptibility have been suggested to contribute to the geographic distribution of H. tvaerminnensis.[8]
Morphology and life cycle
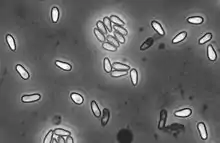
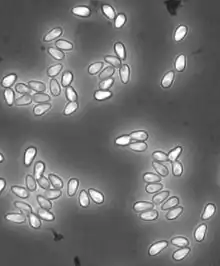
H. magnivora reproduces sexually, while H. tvaerminnensis has an obligatory asexual status.[9] All stages of vegetative reproduction (merogony) are enclosed by a thick plasma membrane, which is in direct contact with the cytoplasma of the host cell. In all stages the nuclei is isolated and clearly visible. The onset of the sporogony is the production of a sporophorous vesicle that is connected to the plasma membrane by tubules. Then fabelliform budding of mostly eight sporoblasts into the vesicle is initiated. Initially the wall of the encysted spore (sporont) has two layers. During the maturating of the sporont two generations of tubules are present in the episporontal space, being reduced at maturity. Mature spores of the genus Hamiltosporidium are polymorph, though pyriform and elongated rod-like spores dominate in H. tvaerminnensis[2] while H. magnivora has lightly pyriform spores only.[5] Pyriform spores measure 4·9–5·6 x 2·2–2·3 μm while elongated spores measure 6·8–12·0 x 1·6–2·1 μm in H. tvaerminnensis.[10] Lightly pyriform spores in H. magnivora measure 2.34-3.03 x 4.07-4.93 μm.[5] The polar filaments in the mature spore are more or less isofilar and the polaroplast has 2 – 3 distinct lamellar regions.[2]
Infection
The unicellular parasites infect the crustacean Daphnia magna. Both species H. magnivora and H. tvaerminnensis infect the fat body, the hypodermis and the ovaries of the crustacean.[5][10] Infection can be detected by examining crushed animals under a phase contrast microscope (400x). In advanced stages of the infection spores are found in the whole body cavity of host animals, which leads to a clearly visible whitish coloration. The infection leads to reduction in the host fitness by reducing life expectancy, fecundity and competitive ability for resources among D. magna.[11][12]
Transmission
In H. tvaerminnensis horizontal transmission occurs after the host's death, when spores from decaying cadaver suspend in the water. Spores can persist in the environment, being able to infect new hosts after extended periods of time.[2] Horizontal transmission could not be observed in the lab for H. magnivora but it is suggested to happen in nature as witnessed in H. tvaerminnensis.[6] Both parasite species are vertically transmitted to asexual host offspring with a transmission rate of up to 100% and a somewhat lower transmission rate to sexual offspring (transmission rates have only been reported for H. tvaerminnensis).[13] This means that the parasite is hardly lost in a clone lineage and also survives inside the resting eggs of its host. Thereby it can be kept in a population even though the host is resting while the habitat is dried up or frozen. Because of its combined vertical and horizontal transmission strategies, H. tvaerminnensis can reach prevalence of up to 100% in asexual populations in nature as well as in the laboratory.[12][14]
Genome
In 2009 a first draft of H. tvaerminnensis genome was sequenced (under the name of Octosporea bayeri).[15] The whole genome has approximately the size of 24.2 Mega-bases (Mb). This stands in contrast to other sequenced microsporidia genomes (for example the compact 2.9 Mb genome of the obligate parasite Encephalitozoon cuniculi[16]) which are reduced in size. It is suggested that H. tvaerminnensis genome is the largest known microsporidian genome. Almost half of the genome sequence was found to be unique for H. tvaerminnensis. Compared to smaller microsporidian genome, Gene density is rather low in H. tvaerminnensis but variable.[15]
The H. tvaerminnensis population in the Baltic Sea is genetically very homogeneous. So far there were only two main haplotype found in the whole population. Comparison of the genetic diversity to its sister species H. magnivora revealed H. tvaerminnensis asexuality.[9]
References
- "Hamiltosporidium". www.uniprot.org. Retrieved 2016-03-05.
- Haag, Karen Luisa; Larsson, J. I. Ronny; Refardt, Dominik; Ebert, Dieter (2011-04-01). "Cytological and molecular description of Hamiltosporidium tvaerminnensis gen. et sp. nov., a microsporidian parasite of Daphnia magna, and establishment of Hamiltosporidium magnivora comb. nov" (PDF). Parasitology. 138 (4): 447–462. doi:10.1017/S0031182010001393. ISSN 1469-8161. PMID 20946698.
- Altermatt, F.; Hottinger, F.W. & D. Ebert (2007). "Parasites promote host gene flow in a metapopulation" (PDF). Evolution Ecology. 21 (4): 561–575. doi:10.1007/s10682-006-9136-6.
- Zbinden, M.; Haag, C. R.; Ebert, D. (2008-07-01). "Experimental evolution of field populations of Daphnia magna in response to parasite treatment". Journal of Evolutionary Biology. 21 (4): 1068–1078. doi:10.1111/j.1420-9101.2008.01541.x. ISSN 1420-9101. PMID 18462312.
- Larsson J.I.R.; D. Ebert; K.L. Mangin; J. Vavra (1998). "Ultrastructural study and description of Flabelliforma magnivora sp. n. (Microspora: Duboscqiidae), a microsporidian parasite of Daphnia magna (Crustacea: Cladocera: Daphniidae)". Acta Protozoologica. 37: 41–52.
- Mangin, K. L.; M. Lipsitch & D. Ebert (1995). "Virulence and transmission modes of two microsporidia in Daphnia magna". Parasitology. 111 (2): 133–142. doi:10.1017/s0031182000064878.
- Haag, K. L.; Sheikh-Jabbari, E.; Ben-Ami, F.; Ebert, D. (2013-05-01). "Microsatellite and single-nucleotide polymorphisms indicate recurrent transitions to asexuality in a microsporidian parasite". Journal of Evolutionary Biology. 26 (5): 1117–1128. doi:10.1111/jeb.12125. ISSN 1420-9101. PMID 23530861.
- Lange, Benjamin; Kaufmann, Andrea Patricia; Ebert, Dieter (2015-11-01). "Genetic, ecological and geographic covariables explaining host range and specificity of a microsporidian parasite". The Journal of Animal Ecology. 84 (6): 1711–1719. doi:10.1111/1365-2656.12421. ISSN 1365-2656. PMID 26147623.
- Haag, Karen L.; Traunecker, Emmanuel; Ebert, Dieter (2013-01-01). "Single-nucleotide polymorphisms of two closely related microsporidian parasites suggest a clonal population expansion after the last glaciation". Molecular Ecology. 22 (2): 314–326. doi:10.1111/mec.12126. ISSN 1365-294X. PMID 23163569.
- Vizoso, D. B.; Ebert, D. (2005-07-01). "Phenotypic plasticity of host-parasite interactions in response to the route of infection" (PDF). Journal of Evolutionary Biology. 18 (4): 911–921. doi:10.1111/j.1420-9101.2005.00920.x. ISSN 1010-061X. PMID 16033563.
- Ben-Ami, F.; Rigaud, T.; Ebert, D. (2011-06-01). "The expression of virulence during double infections by different parasites with conflicting host exploitation and transmission strategies". Journal of Evolutionary Biology. 24 (6): 1307–1316. doi:10.1111/j.1420-9101.2011.02264.x. ISSN 1420-9101. PMID 21481055.
- Bieger, Annette; Ebert, Dieter (2009-05-01). "Expression of parasite virulence at different host population densities under natural conditions" (PDF). Oecologia. 160 (2): 247–255. Bibcode:2009Oecol.160..247B. doi:10.1007/s00442-009-1297-x. ISSN 1432-1939. PMID 19219457.
- Vizoso, D. B.; Lass, S.; Ebert, D. (2005-05-01). "Different mechanisms of transmission of the microsporidium Octosporea bayeri: a cocktail of solutions for the problem of parasite permanence" (PDF). Parasitology. 130 (Pt 5): 501–509. doi:10.1017/s0031182004006699. ISSN 0031-1820. PMID 15991493.
- Ebert, D. (2005). Ecology, epidemiology and evolution of parasitism in Daphnia. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information.
- Corradi, Nicolas; Haag, Karen L.; Pombert, Jean-François; Ebert, Dieter; Keeling, Patrick J. (2009-01-01). "Draft genome sequence of the Daphnia pathogen Octosporea bayeri: insights into the gene content of a large microsporidian genome and a model for host-parasite interactions". Genome Biology. 10 (10): R106. doi:10.1186/gb-2009-10-10-r106. ISSN 1474-760X. PMC 2784321. PMID 19807911.
- Katinka, Michaël D.; Duprat, Simone; Cornillot, Emmanuel; Méténier, Guy; Thomarat, Fabienne; Prensier, Gérard; Barbe, Valérie; Peyretaillade, Eric; Brottier, Philippe (2001-11-22). "Genome sequence and gene compaction of the eukaryote parasite Encephalitozoon cuniculi". Nature. 414 (6862): 450–453. Bibcode:2001Natur.414..450K. doi:10.1038/35106579. ISSN 0028-0836. PMID 11719806.
Further reading
- Ebert, D. (2005). Ecology, epidemiology and evolution of parasitism in Daphnia. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information.