Hexachlorophosphazene
Hexachlorophosphazene is an inorganic compound with the formula (NPCl2)3. The molecule has a cyclic, unsaturated backbone consisting of alternating phosphorus and nitrogen centers. Its classification as a phosphazene highlights its relationship to benzene.[1] It can be viewed as a trimer of the hypothetical compound N≡PCl2.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Hexachlorotriphosphazene | |
| Other names
Phosphonitrilic chloride Hexachlorocyclotriphosphazene Triphosphonitrilic chloride 2,2,4,4,6,6-hexachloro-2,2,4,4,6,6- hexahydro-1,3,5,2,4,6-triazatriphosphorine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.012.160 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| N3Cl6P3 | |
| Molar mass | 347.66 g/mol |
| Appearance | colorless solid |
| Density | 1.98 g/mL at 25 °C |
| Melting point | 112 to 115 °C (234 to 239 °F; 385 to 388 K) |
| Boiling point | decomposes |
| decomposes | |
| Solubility in chlorocarbons | soluble |
| Structure | |
| 0 D | |
| Hazards | |
| Main hazards | mild irritant |
| GHS pictograms |  |
| GHS Signal word | Danger |
| H314 | |
| P260, P264, P280, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P321, P363, P405, P501 | |
| Flash point | Non-flammable |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
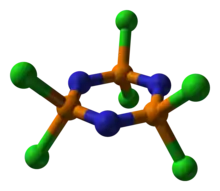
Structure
Chemists have long known of rings containing purely carbon or carbon and other elements, e.g. benzene, pyridine, or cyclohexane. Related cyclic compounds lacking in carbon have also been studied; hexachlorotriphosphazene is one such inorganic ring. Other well known inorganic ring systems include borazine, the bicyclic S4N4, and the cyclic siloxanes.
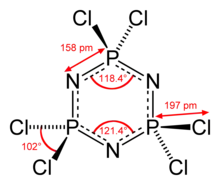
The molecule has a P3N3 core with six equivalent P-N bonds. The molecule has D3h symmetry. The P-N distances are 157 pm. Each phosphorus center is tetrahedral with Cl-P-Cl angle of 101°.[2] The P3N3 ring nearly planar in hexachlorophosphazene but is strictly planar in hexafluorophosphazene.[3]
Bonding
Early analyses
Cyclophosphazenes such as hexachlorotriphosphazene are distinguished by notable stability and equal P-N bond lengths, which imply delocalization and perhaps even aromaticity. To account for these features, early bonding models invoked a delocalised π system arising from the overlap of N 2p and P 3d orbitals.[3]
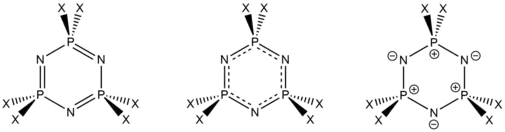
Modern bonding models
Modern calculations reveal that the P 3d contribution is negligible, invalidating the earlier hypothesis; instead a charge separated model is more generally accepted.[1] According to this description, the P–N bond is viewed as a very polarised one (between notional P+ and N–, as in the right), with sufficient ionic character to account for most of the bond strength.
The rest (~15%) of the bond strength may be attributed to a negative hyperconjugation interaction: the N lone pairs can donate some electron density into π-accepting σ* molecular orbitals on the P.
Synthesis
The original synthesis involved the reaction of phosphorus pentachloride and ammonia or ammonium chloride, yielding a mixture of products.[3] When conducted in hot chlorocarbon solvents, the following stoichiometry applies:
- NH4Cl + PCl5 → 1/n (Cl2PN)n + HCl
where n can usually take values of n=2 (the dimer tetrachlorodiphosphazene), n=3 (the trimer hexachlorotriphosphazene), and n=4 (the tetramer octachlorotetraphosphazene).[4]
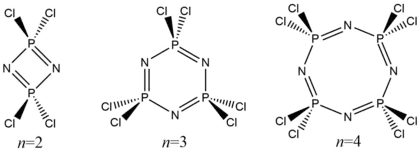
Purification by sublimation gives mainly the trimer and tetramer, while slow sublimation under vacuum at approximately 60 °C affords the pure trimer free of the tetramer. Reaction conditions such as temperature may also be tuned to maximise the yield of the trimer at the expense of the other possible products; nonetheless, commercial samples of hexachlorotriphosphazene usually contain appreciable amounts of octachlorotetraphosphazene, even up to 40%.[5]
Reactions
Substitution at P
Hexachlorophosphazene reacts readily with oxygen and nitrogen nucleophiles.[1][3] Alkali metal alkoxides and amides are commonly used. The susceptibility of the P-Cl bonds towards substitution is highly distinctive.[6]
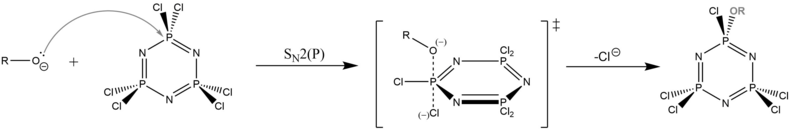
The polysubstitution of the initial phosphazene raises the issue of regioselectivity. The dominant factors affecting this are found to be: (a) steric effects; and (b) the ability of the alkoxy oxygen lone pair to π-backdonate to the P atom where substitution has occurred, decreasing the latter's electrophilicity and deactivating it towards further substitution.[1] In combination, these factors usually lead to strong preference for 2,4 over a 2,2 attack of monosubstituted intermediates (note numbering of ring positions starts from N). These intermediates go on to form 2,4,6-trisubstituted and 2,2,4,6-tetrasubstituted intermediates before reaching hexasubstitution.[1] The resulting hexalkoxyphosphazenes (especially the aryloxy species) are valued for their high thermal and chemical stability, as well as their low glass transition temperature, which implies flexibility at low temperatures. Some hexalkoxyphosphazenes found commercial use as fireproof materials and high temperature lubricants.[6]
Polymerisation: "Inorganic rubber"
Heating hexachlorophosphazene to ca. 250 °C induces polymerization. The conversion is a type of ring-opening polymerisation. The product is a linear polymer (PNCl2)n, where n ~ 15000. The tetramer also polymerises in this manner, although more slowly.[6]
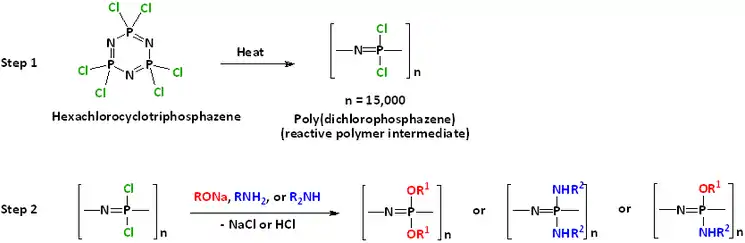
The resulting inorganic chloropolymer is the starting point for a wide class of polymeric derivatives, collectively known as polyphosphazenes. Substitution of the chloride groups by other nucleophilic groups, especially alkoxides as laid out above, yields numerous characterised derivatives. Some of these polyphosphazenes appear promising for potential commercial applications such as high performance elastomers or thermoplastics with various desirable properties.
Lewis basicity
The nitrogen centers are mildly basic. Hexachlorophosphazene forms a 1:1 adduct with aluminium trichloride.[7]
Other reactions
The trimer has also found applications in research by enabling aromatic coupling reactions between pyridine and either N,N-dialkylanilines or indole, resulting in 4,4'-substituted phenylpyridine derivatives, postulated to go through a cyclophosphazene pyridinium salt intermediate.[5]
The compound may also be used as a peptide coupling reagent for the synthesis of oligopeptides in chloroform, though for this application the tetramer octachlorotetraphosphazene usually proves more effective.[5]
Finally, both the trimer and tetramer may photochemically react when dissolved in hexane, decaline, benzene or toluene, forming clear liquids identified as alkyl-substituted at P derivatives of the form (NPCl2-xRx)n=3,4.[5] Such reactions proceed under prolonged UVC (mercury arc) illumination, yet the cyclophosphazene rings are not affected, a testiment of their notable chemical stability. Indeed, solid films of the trimer and tetramer will not undergo any chemical change under such irradiation conditions when not in solution.
Further reading
- Discovery of phosphazeness: Liebig-Wöhler, Briefwechsel vol. 1, 63; Ann. Chem. (Liebig), vol. 11 (1834), 146.
- First reports on their polymerization: H. N. Stokes (1895), On the chloronitrides of phosphorus. American Chemical Journal, vol. 17, p. 275.H. N. Stokes (1896), On Trimetaphosphimic acid and its decomposition products. American Chemical Journal, vol. 18 issue 8, p. 629.
- Allcock, Harry R.; Ngo, Dennis C.; Parvez, Masood; Whittle, Robert R.; Birdsall, William J. (1991-03-01). "Syntheses and structures of cyclic and short-chain linear phosphazenes bearing 4-phenylphenoxy side groups". Journal of the American Chemical Society. 113 (7): 2628–2634. doi:10.1021/ja00007a041. ISSN 0002-7863.
- Ye, Chengfeng; Zhang, Zefu; Liu, Weimin (2002-01-01). "A Novel Synthesis of Hexasubstituted Cyclotriphosphazenes". Synthetic Communications. 32 (2): 203–209. doi:10.1081/SCC-120002003. ISSN 0039-7911. S2CID 97319633.
References
- Allen, Christopher W. (1991-03-01). "Regio- and stereochemical control in substitution reactions of cyclophosphazenes". Chemical Reviews. 91 (2): 119–135. doi:10.1021/cr00002a002. ISSN 0009-2665.
- Bartlett, Stewart W.; Coles, Simon J.; Davies, David B.; Hursthouse, Michael B.; i̇Bişogˇlu, Hanife; Kiliç, Adem; Shaw, Robert A.; Ün, İlker (2006). "Structural investigations of phosphorus–nitrogen compounds. 7. Relationships between physical properties, electron densities, reaction mechanisms and hydrogen-bonding motifs of N3P3Cl(6 − n)(NHBu t ) n derivatives". Acta Crystallographica Section B Structural Science. 62 (2): 321–329. doi:10.1107/S0108768106000851. PMID 16552166.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- Allcock, H. R. (1972). Phosphorus-nitrogen compounds ; cyclic, linear, and high polymeric systems. New York: Academic Press. ISBN 978-0-323-14751-4. OCLC 838102247.
- Mark, J. E.; Allcock, H. R.; West, R. “Inorganic Polymers” Prentice Hall, Englewood, NJ: 1992. ISBN 0-13-465881-7.
- Heston, Amy J.; Panzner, Matthew J.; Youngs, Wiley J.; Tessier, Claire A. (2005). "Lewis Acid Adducts of [PCl2N]3". Inorganic Chemistry. 44 (19): 6518–6520. doi:10.1021/ic050974y. PMID 16156607.
| Wikimedia Commons has media related to hexachlorophosphazene. |