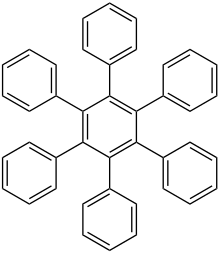
Hexaphenylbenzene
Hexaphenylbenzene is an aromatic compound composed of a benzene ring substituted with six phenyl rings. It is a colorless solid. The compound is the parent member of a wider class of hexaarylbenzenes, which are mainly of theoretical interest.[2]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
1,2,3,4,5,6-hexa(phenyl)benzene | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.012.356 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C42H30 | |
| Molar mass | 534.702 g·mol−1 |
| Appearance | white solid |
| Melting point | 454 to 456 °C (849 to 853 °F; 727 to 729 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
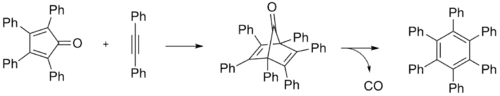
It is prepared by heating tetraphenylcyclopentadienone and diphenylacetylene in benzophenone or other high-temperature solvent. The reaction proceeds via a Diels-Alder reaction to give the hexaphenyldienone, which then eliminates carbon monoxide.[1]
Together with 1,2,3,4-tetraphenylnaphthalene, hexaphenylbenzene forms by the chromium-catalyzed oligomerization of diphenylacetylene.[3]
Structure
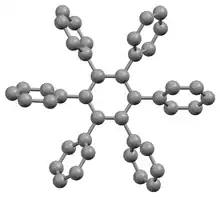
The stable conformation of this molecule has the phenyl rings rotated out of the plane of the central benzene ring. The molecule adopts a propeller-like conformation in which the phenyl rings are rotated about 65°,[4] while in the gas phase, they are perpendicular with some slight oscillations.[5]
References
- Louis Fieser (1966). "Hexaphenylbenzene". Organic Syntheses. 46: 44. doi:10.15227/orgsyn.046.0044.
- Varun Vij, Vandana Bhalla, and Manoj Kumar (2016). "Hexaarylbenzene: Evolution of Properties and Applications of Multitalented Scaffold". Chemical Reviews. 116 (16): 9565–9627. doi:10.1021/acs.chemrev.6b00144. PMID 27498592.CS1 maint: multiple names: authors list (link)
- W. Herwig, W. Metlesics, H. Zeiss (1959). "π-Complexes of the Transition Metals. X. Acetylenic π-Complexes of Chromium in Organic Synthesis". J. Am. Chem. Soc. 81 (23): 6203–6207. doi:10.1021/ja01532a024.CS1 maint: multiple names: authors list (link)
- Bart, J. C. J. (1968). "The crystal structure of a modification of hexaphenylbenzene" (PDF). Acta Crystallographica Section B. 24 (10): 1277–1287. doi:10.1107/S0567740868004176.
- Gust, D. (1977). "Restricted Rotation in Hexaarylbenzenes". J. Am. Chem. Soc. 99 (21): 6980–6982. doi:10.1021/ja00463a034.