Tetraphenylcyclopentadienone
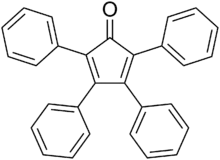
Tetraphenylcyclopentadienone is an organic compound with the formula (C6H5)4C4CO. It is a dark purple to black crystalline solid that is soluble in organic solvents. It is an easily made building block for many organic and organometallic compounds.
 | |
 Perspective view, showing the canted phenyl rings[1] | |
 | |
| Names | |
|---|---|
| IUPAC name
2,3,4,5-Tetraphenyl-2,4- cyclopentadien-1-one | |
| Other names
Tetracyclone, TPCPD, Cyclone | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.006.847 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C29H20O | |
| Molar mass | 384.478 g·mol−1 |
| Melting point | 219 to 220 °C (426 to 428 °F; 492 to 493 K)[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Structure
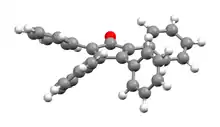
The C5O core of the molecule is planar and conjugated, but the bonds have a definite alternating single- and double-bond nature. The C2–C3 and C4–C5 distances are 1.35 Å, while the C1–C2, C3–C4, C5–C1 are closer to single bonds with distances near 1.50 Å.[1] The phenyl groups of tetraphenylcyclopentadienone adopt a "propeller" shape in its 3D conformation. The four phenyl rings are rotated out of the plane of the central ring because of steric repulsion with each other.[3]
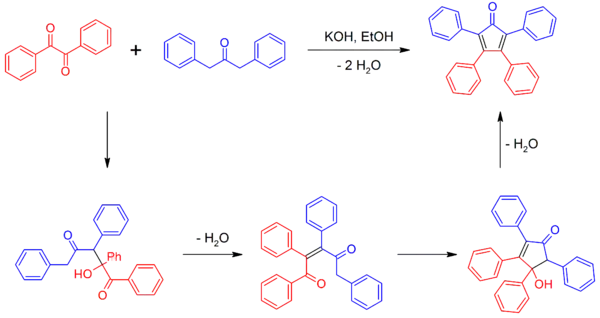
Synthesis
Tetraphenylcyclopentadienone can be synthesized by a double aldol condensation involving benzil and dibenzyl ketone in the presence of a basic catalyst.[2][4]
Reactions
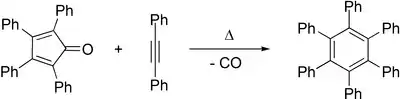
The central ring can act as a diene in Diels–Alder reactions with various dienophiles. For example, reaction with benzyne leads to 1,2,3,4-tetraphenylnaphthalene and reaction with diphenylacetylene leads to hexaphenylbenzene.[4] In this way, it is a precursor to graphene-like molecules,[5] such as coronene.
Similarly, pentaphenylpyridine derivatives may be prepared via a Diels–Alder reaction between tetraphenylcyclopentadienone and benzonitrile.
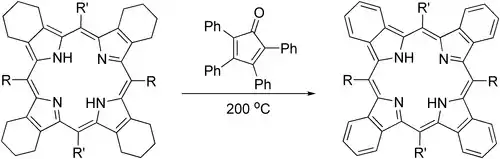
Tetraphenylcyclopentadienone can provide an effective alternative to DDQ in aromatization of parts of porphyrin structures:[6]
Ligand in organometallic chemistry
Tetraarylcyclopentadienones are a well studied class of ligands in organometallic chemistry. The Shvo catalyst, useful for certain hydrogenations, is derived from tetraphenylcyclopentadienone.[7]

See also
References
- J. C. Barnes; W. M. Horspool; F. I. Mackie (1991). "2,3,4,5-Tetraphenylcyclopenta-2,4-dien-1-one and 5,6,7,8-tetrachloro-3a,9a-dihydro-2,3,3a,9a-tetraphenylcyclopenta[2,3-b][1,4]benzodioxin-1-one–toluene (2/1): Compounds of photochemical interest". Acta Crystallogr. C. 47: 164–168. doi:10.1107/S0108270190005145.
- John R. Johnson, J. R.; Grummitt, O. (1943). "Tetraphenylcyclopentadienone". Organic Syntheses. 23: 92.CS1 maint: multiple names: authors list (link); Collective Volume, 3, p. 805
- Sheley, C. F.; Shechter, H. (1970). "Cyclopentadienones from 1,2,4-cyclopentanetriones, 2-cyclopentene-1,4-diones, and 3-cyclopentene-1,2-diones". The Journal of Organic Chemistry. 35 (7): 2367–2374. doi:10.1021/jo00832a058.
- Fieser, L. F. (1966). "Hexaphenylbenzene". Organic Syntheses. 46: 44.; Collective Volume, 5, p. 604
- Feng, Xinliang; Pisula, Wojciech; Müllen, Klaus (31 January 2009). "Large polycyclic aromatic hydrocarbons: Synthesis and discotic organization". Pure and Applied Chemistry. 81 (12): 2203–2224. doi:10.1351/PAC-CON-09-07-07.
- M.A. Filatov; A.Y. Lebedev; S.A. Vinogradov; A.V. Cheprakov (2008). "Synthesis of 5,15-Diaryltetrabenzoporphyrins". J. Org. Chem. 73 (11): 4175–4185. doi:10.1021/jo800509k. PMC 2491715. PMID 18452337.
- Quintard, Adrien; Rodriguez, Jean (14 April 2014). "Iron Cyclopentadienone Complexes: Discovery, Properties, and Catalytic Reactivity". Angewandte Chemie International Edition. 53 (16): 4044–4055. doi:10.1002/anie.201310788. PMID 24644277.