Hexon protein
In molecular biology, the hexon protein is a major coat protein found in Adenoviruses. Hexon coat proteins are synthesised during late infection and form homo-trimers. The 240 copies of the hexon trimer that are produced are organised so that 12 lie on each of the 20 facets. The central 9 hexons in a facet are cemented together by 12 copies of polypeptide IX. The penton complex, formed by the peripentonal hexons and penton base (holding in place a fibre), lie at each of the 12 vertices.[1] The hexon coat protein is a duplication consisting of two domains with a similar fold packed together like the nucleoplasmin subunits. Within a hexon trimer, the domains are arranged around a pseudo 6-fold axis. The domains have a beta-sandwich structure consisting of 8 strands in two sheets with a jelly-roll topology; each domain is heavily decorated with many insertions.[2] Some hexon proteins contain a distinct C-terminal domain.
| Adeno_hexon | |||||||||
|---|---|---|---|---|---|---|---|---|---|
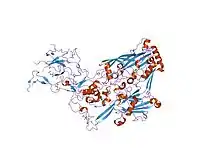 refinement of adenovirus type 2 hexon with cns | |||||||||
| Identifiers | |||||||||
| Symbol | Adeno_hexon | ||||||||
| Pfam | PF01065 | ||||||||
| InterPro | IPR016107 | ||||||||
| SCOP2 | 1dhx / SCOPe / SUPFAM | ||||||||
| |||||||||
| Adeno_hexon_C | |||||||||
|---|---|---|---|---|---|---|---|---|---|
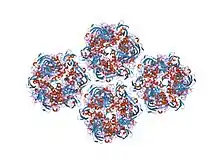 the quasi-atomic model of human adenovirus type 5 capsid (part 2) | |||||||||
| Identifiers | |||||||||
| Symbol | Adeno_hexon_C | ||||||||
| Pfam | PF03678 | ||||||||
| InterPro | IPR016108 | ||||||||
| SCOP2 | 1dhx / SCOPe / SUPFAM | ||||||||
| |||||||||
Hexon directly recruits the cellular motor protein dynein in a pH-dependent manner.[3] The dynein-regulatory protein, dynactin, was found to play a clear role in regulating the dynein-adenovirus complex transport to the nucleus.
References
- Athappilly FK, Murali R, Rux JJ, Cai Z, Burnett RM (September 1994). "The refined crystal structure of hexon, the major coat protein of adenovirus type 2, at 2.9 A resolution". Journal of Molecular Biology. 242 (4): 430–55. doi:10.1006/jmbi.1994.1593. PMID 7932702.
- Rux JJ, Kuser PR, Burnett RM (September 2003). "Structural and phylogenetic analysis of adenovirus hexons by use of high-resolution x-ray crystallographic, molecular modeling, and sequence-based methods". Journal of Virology. 77 (17): 9553–66. doi:10.1128/jvi.77.17.9553-9566.2003. PMC 187380. PMID 12915569.
- Bremner KH, Scherer J, Yi J, Vershinin M, Gross SP, Vallee RB (December 2009). "Adenovirus transport via direct interaction of cytoplasmic dynein with the viral capsid hexon subunit". Cell Host & Microbe. 6 (6): 523–35. doi:10.1016/j.chom.2009.11.006. PMC 2810746. PMID 20006841.
