Hydroxylammonium nitrate
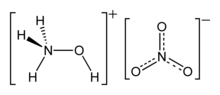
Hydroxylammonium nitrate or hydroxylamine nitrate (HAN) is an inorganic compound with the chemical formula [NH3OH][NO3]. It is a salt derived from hydroxylamine and nitric acid. In its pure form, it is a colourless hygroscopic solid. It has potential to be used as a rocket propellant either as a solution in monopropellants or bipropellants.[1] Hydroxylammonium nitrate (HAN) based propellants are a viable and effective solution for future green propellant based missions, as it offers 50% higher performance for a given propellant tank compared to commercially used hydrazine.
 | |
 | |
| Names | |
|---|---|
| Other names
hydroxylamine nitrate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.033.342 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| H4N2O4 | |
| Molar mass | 96.04 g/mol |
| Density | 1.84 g/cm3 |
| Melting point | 48 °C |
| Soluble | |
| Hazards | |
| Safety data sheet | External MSDS (as 18 % solution) |
EU classification (DSD) (outdated) |
Explosive (E) Carc. Cat. 3 Toxic (T) Harmful (Xn) Irritant (Xi) Dangerous for the environment (N) |
| R-phrases (outdated) | R2, R22, R24, R36/38, R40, R43, R48/22, R50 |
| S-phrases (outdated) | (S1/2), S26, S36/37, S45, S61 |
| Related compounds | |
Other anions |
Hydroxylammonium sulfate Hydroxylammonium chloride |
Other cations |
Ammonium nitrate |
Related compounds |
Hydroxylamine |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Properties
The compound is a salt with separated hydroxyammonium and nitrate ions.[2] Hydroxylammonium nitrate is unstable because it contains both a reducing agent (hydroxylammonium cation) and an oxidizer (nitrate),[3] the situation being analogous to ammonium nitrate. It is usually handled as an aqueous solution. The solution is corrosive and toxic, and may be carcinogenic. Solid HAN is unstable, particularly in the presence of trace amounts of metal salts.
Laboratory preparatory routes
- Double Decomposition
- Neutralization
- Ion exchange via resins
- Electrolysis
- Hydrogenation of nitric acid
- Catalytic Reduction of nitric oxides
Applications
HAN has applications as a component of rocket propellant, in both solid and liquid form. HAN and ammonium dinitramide (ADN), another energetic ionic compound, were investigated as less-toxic replacements for toxic hydrazine for monopropellant rockets where only a catalyst is needed to cause decomposition.[4] HAN and ADN will work as monopropellants in water solution, as well as when dissolved with fuel liquids such as glycine or methanol.
HAN is used by the Network Centric Airborne Defense Element boost-phase interceptor being developed by Raytheon.[5] As a solid propellant oxidizer, it is typically bonded with glycidyl azide polymer (GAP), hydroxyl-terminated polybutadiene (HTPB), or carboxy-terminated polybutadiene (CTPB) and requires preheating to 200-300 °C to decompose. When used as a monopropellant, the catalyst is a noble metal, similar to the other monopropellants that use silver, palladium, or iridium.
HAN also enabled the development of solid propellants that could be controlled electrically and switched on and off.[6] Developed by DSSP for special effects[7] and microthrusters, these were the first HAN based propellants in space; and aboard the Naval Research Laboratory SpinSat, launched in 2014.[8][9]
It will be used in a fuel/oxidizer blend known as "AF-M315E"[10] in the high thrust engines of the Green Propellant Infusion Mission,[11][12][13] which was initially expected to be launched in 2015, and eventually launched and deployed on 25 June 2019.[14] The specific impulse of AF-M315E is 257 s.[1] The aqueous solution of HAN can be added with fuel components such as methanol, glycine, TEAN (tri-ethanol-ammonium nitrate) and amines to form best high performance monopropellants for space propulsion systems.
China Aerospace Science and Technology Corporation (CASC) launched a demonstration of HAN-based thruster aboard a microsatellite in January 2018.[15]
Japanese technology demonstration satellite Innovative Satellite Technology Demonstration-1, launched in January 2019, contains a demonstration thruster using HAN and operated successfully in orbit.[16][17]
HAN is sometimes used in nuclear reprocessing as a reducing agent for plutonium ions.
Bibliography
- Donald G. Harlow et al. (1998). "Technical Report on Hydroxlyamine Nitrate". U.S. Department of Energy. DOE/EH-0555
- Gösta Bengtsson et al. (2002) "The kinetics and mechanism of oxidation of hydroxylamine by iron(III)". J. Chem. Soc., Dalton Trans., 2002, 2548–2552
References
- Spores, Ronald A.; Masse, Robert; Kimbrel, Scott; McLean, Chris (15–17 July 2013). "GPIM AF-M315E Propulsion System" (PDF). San Jose, California, USA: 49th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit. Archived (PDF) from the original on 2014-02-28.
- Rheingold, A. L.; Cronin, J. T.; Brill, T. B.; Ross, F. K. (March 1987). "Structure of hydroxylammonium nitrate (HAN) and the deuterium homolog". Acta Crystallographica Section C. 43 (3): 402–404. doi:10.1107/S0108270187095593.
- Pembridge, John R.; et al. (1979). Kinetics, Mechanism, and Stoichiometry of the Oxidation of Hydroxylamine by Nitric Acid. JCS Dalton. pp. 1657–1663.
- Dominic Freudenmann, Helmut K. Ciezki (29 July 2019). "ADN and HAN‐Based Monopropellants – A Minireview on Compatibility and Chemical Stability in Aqueous Media". Propellants, Explosives, Pyrotechnics. Wiley Online Library. 44 (9): 1084–1089. doi:10.1002/prep.201900127.CS1 maint: uses authors parameter (link)
- "Boost phase interceptor". Press Releases. Raytheon. Archived from the original on May 18, 2007.
- Sawka, Wayne N.; McPherson, Michael (2013-07-12), "Electrical Solid Propellants: A Safe, Micro to Macro Propulsion Technology", 49th AIAA/ASME/SAE/ASEE Joint Propulsion Conference, Joint Propulsion Conferences, American Institute of Aeronautics and Astronautics, doi:10.2514/6.2013-4168, ISBN 978-1-62410-222-6
- "LDI 2014 Award Winners Announced". Live Design. 2014-11-23. Retrieved 2019-06-19.
- Nicholas, Andrew; Finne, Ted; Gaylsh, Ivan; Mai, Anthony; Yen, Jim (September 2013). "SpinSat Mission Overview" (PDF).
- "SpinSat - Satellite Missions - eoPortal Directory". directory.eoportal.org. Retrieved 2019-06-19.
- Spores, Ronald A.; Robert Masse, Scott Kimbrel, Chris McLean (15–17 July 2013), "GPIM AF-M315E Propulsion System" (PDF), 49th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, San Jose, California, USACS1 maint: multiple names: authors list (link)
- "About Green Propellant Infusion Mission (GPIM)". NASA. 2014. Archived from the original on 2013-04-24.
- "Green Propellant Infusion Mission (GPIM)". Ball Aerospace. 2014. Archived from the original on 2013-04-24.
- Casey, Tina (19 July 2013). "NASA Sets Its Sights On $45 Million Green Fuel Mission". Clean Technica.
- Sempsrott, Danielle (25 June 2019). "NASA's Green Propellant Infusion Mission Deploys". NASA. Retrieved 6 June 2020.
- 航天科技六院801所HAN 基无毒推进发动机研制攻关记 (in Chinese). China Aerospace Science and Technology Corporation. 24 May 2019. Retrieved 14 May 2020.
- "革新的衛星技術実証1号機 PRESS KIT" (PDF). JAXA. Retrieved 15 March 2019.
- 小型実証衛星1号機 RAPIS-1 グリーンプロペラント推進系(GPRCS)世界初の軌道上 HAN系推進薬 実証! (in Japanese). JAXA. 15 March 2019. Retrieved 15 March 2019.