Iodate
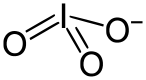
An iodate is the anion with the formula IO−
3. It is the most common form of iodine in nature, as it comprises the major iodine-containing ores.[1] Iodide salts are often colorless.
3

Structure
Iodate is pyramidal in structure. The O-I-O angles range from 105-97°, somewhat smaller than the O-Cl-O angles in chlorate.[2]
Reactions
Redox
Iodate is one of several oxyanions of iodine. It participates in several redox reactions, e.g. the iodine clock reaction. Iodate show no tendency to disproportionate to periodate and iodide, in contrast to the situation for chlorate.
Iodate is reduced by sulfite:[1]
- 6 HSO3- + 2 IO3- → 2 NaI + 6 HSO4-
Iodate oxidizes iodide:
- 5 I- + IO3- + 3 H2SO4 → 3 I2 + 3 H2O + 3 SO42-
Similarly chlorate oxidizes iodide:
- I- + ClO3- → Cl- + IO3-
Iodate is also obtained by reducing a periodate with a sulfide. The byproduct of the reaction is a sulfoxide.[3]
Acid-base
Iodate is unusual in that it forms a strong hydrogen bond with its parent acid:[2]
- IO3- + HIO3 → H[IO3]2-
The anion H[IO3]2- is referred to as biiodate.
Principle compounds
- Calcium iodate (Ca(IO3)2), the principal ore of iodine. It is also used as a nutrional supplement for cattle.
- Potassium iodate, like potassium iodide, has been issued as a prophylaxis against radioiodine absorption in some countries.[4][5] Potassium hydrogen iodate (KH(IO3)2) is a double salt of potassium iodate and iodic acid and an acid as well.
Natural occurrence
Minerals containing iodate are found in the caliche deposits of Chile. The most important iodate minerals are lautarite and brüggenite, but also copper-bearing iodates (e.g., salesite) are known.[6]
References
- Lyday, Phyllis A. (2005). "Iodine and Iodine Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. pp. 382–390. doi:10.1002/14356007.a14_381.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Qiu, Chao; Sheng Han; Xingguo Cheng; Tianhui Ren (2005). "Distribution of Thioethers in Hydrotreated Transformer Base Oil by Oxidation and ICP-AES Analysis" (abstract). Industrial & Engineering Chemistry Research. 44 (11): 4151–4155. doi:10.1021/ie048833b. Retrieved 2007-05-03.
Thioethers can be oxidized to sulfoxides by periodate, and periodate is reduced to iodate
- "Archived copy". Archived from the original on 2013-10-17. Retrieved 2013-04-08.CS1 maint: archived copy as title (link)
- "Archived copy". Archived from the original on 2013-10-18. Retrieved 2013-05-22.CS1 maint: archived copy as title (link)
- http://www.mindat.org
