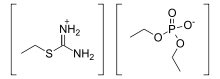
Isothiouronium
In organic chemistry, isothiouronium is a functional group with the formula [RSC(NH2)2]+ (R = alkyl, aryl) and is the acid salt of isothiourea. The H centres can also be replaced by alkyl and aryl. Structurally, these cations resemble guanidinium cations. The CN2S core is planar and the C-N bonds are short.[1]
Synthesis
Salts comprising these anions are typically prepared by alkylation of thiourea:
- SC(NH2)2 + RX → [RSC(NH2)2]+X−
Reactions
Hydrolysis of isothiouronium salts gives thiols.[2]
- [RSC(NH2)2]+X− + NaOH → RSH + OC(NH2)2 + NaX
Isothiouronium salts in which the sulfur has been alkylated, such as S-methylisothiourea hemisulfate (CAS No: 867-44-7), will convert amines into guanidinium groups. This approach is sometimes called the Rathke synthesis [3] after Bernhard Rathke[4] who first reported it in 1881.[5]
- RNH2 + [MeSC(NH2)2]+X− → RNC(NH2)2]+X− + MeSH
Chelating resins with isothiouronium groups are used to recover mercury and noble metals including platinum from solutions.[6]
References
- Barker, J.; Powell, H. R. (1998). "S-Benzylisothiouronium Chloride". Acta Crystallographica Section C. 54 (12): 2019. doi:10.1107/S0108270198008166.
- Helmer Kofod (1963). "Furfuryl Mercaptan". Organic Syntheses. 4: 13.; Collective Volume, 1, p. 66
- Palmer, David C. (2001). "S-Methylisothiourea". e-EROS Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rm199s.
- "Heinrich Bernhard Rathke. (1840-1923)". Berichte der deutschen chemischen Gesellschaft (A and B Series). 57 (9): A83–A92. 8 October 1924. doi:10.1002/cber.19240570929.
- Rathke, B. (July 1881). "Ueber Derivate und Constitution des Schwefelharnstoffs" (PDF). Berichte der deutschen chemischen Gesellschaft. 14 (2): 1774–1780. doi:10.1002/cber.18810140247.
- "Purolite S920 Isothiouronium Chelating Resin". Purolite.
