Kolbe–Schmitt reaction
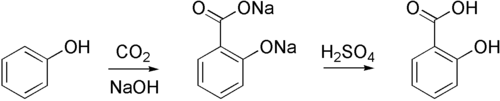
The Kolbe–Schmitt reaction or Kolbe process (named after Hermann Kolbe and Rudolf Schmitt) is a carboxylation chemical reaction that proceeds by heating sodium phenoxide (the sodium salt of phenol) with carbon dioxide under pressure (100 atm, 125 °C), then treating the product with sulfuric acid. The final product is an aromatic hydroxy acid which is also known as salicylic acid (the precursor to aspirin).[1][2][3][4]
| Kolbe–Schmitt reaction | |
|---|---|
| Named after | Hermann Kolbe Rudolf Schmitt |
| Reaction type | Addition reaction |
| Identifiers | |
| Organic Chemistry Portal | kolbe-schmitt-reaction |
| RSC ontology ID | RXNO:0000182 |
By using potassium hydroxide, 4-hydroxybenzoic acid is accessible, an important precursor for the versatile paraben class of biocides used e.g. in personal care products.
The methodology is also used in the industrial synthesis of 3-hydroxy-2-naphthoic acid. The regiochemistry of the carboxylation in this case is sensitive to temperature.[5]
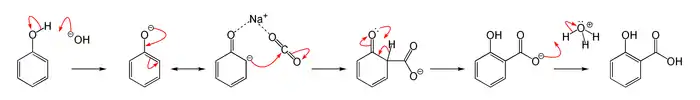
Reaction mechanism
The Kolbe–Schmitt reaction proceeds via the nuecliophillic addition of a phenoxide, classically sodium phenoxide (NaOC6H5), to carbon dioxide to give the salicylate. The final step is reaction of the salicylate with acid to form the desired salicylic acid.
References
- Hermann Kolbe (1860). "Ueber Synthese der Salicylsäure" [On the synthesis of salicylic acid]. Annalen der Chemie und Pharmacie. 113 (1): 125–127. doi:10.1002/jlac.18601130120.
- R. Schmitt (1885). "Beitrag zur Kenntniss der Kolbe'schen Salicylsäure Synthese" [Contribution to [our] knowledge of Kolbe's synthesis of salicylic acid]. Journal für Praktische Chemie. 2nd series. 31 (1): 397–411. doi:10.1002/prac.18850310130.
- A. S. Lindsey and H. Jeskey (1957). "The Kolbe-Schmitt Reaction". Chem. Rev. 57 (4): 583–620. doi:10.1021/cr50016a001. (Review)
- R. T. Morrison and R. N. Boyd (1983). Organic Chemistry (4th ed.). Allyn and Bacon. p. 976-7. ISBN 0-205-05838-8.
- Gerald Booth (2005). "Naphthalene Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_009. ISBN 3527306730..