Legume lectin
The legume lectins (or L-type lectins) are a family of sugar-binding proteins or lectins found in the seeds and, in smaller amounts, in the roots, stems, leaves and bark of plants of the family Fabaceae.[2][3] The exact function of the legume lectins in vivo is unknown but they are probably involved in the defense of plants against predators. Related proteins in other plant families and in animals have also been found. They have been used for decades as a model system for the study of protein-carbohydrate interactions, because they show an amazing variety of binding specificities and are easy to obtain and purify. Over the years, a quite impressive amount of structural data has been gathered.[3] Well-studied members of this protein family include phytohemagglutinin and concanavalin A.
Sugar binding by legume lectins
The legume lectins use an ingenious framework for binding specific sugars. This framework consists of a conserved monosaccharide binding site in which four conserved residues from four separate regions in the protein confer affinity (see figure), a variable loop that confers monosaccharide specificity and a number of subsites around the monosaccharide binding site that harbour additional sugar residues or hydrophobic groups.[3]
Quaternary structure
The legume lectins are also interesting from the point of view of protein structure. Despite the conserved structure of the legume lectin subunit, they can adopt a wide range of quaternary structures.[4][5] The reason behind this remarkable variability is probably to be found in the interaction with multivalent ligands.[6]
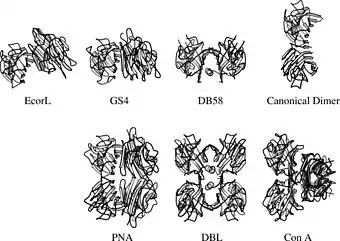 Quaternary structures of some legume lectins. One of the subunits is in the same orientation in all structures for ease of comparison. |
References
- Loris R, Casset F, Bouckaert J, et al. (December 1994). "The monosaccharide binding site of lentil lectin: an X-ray and molecular modelling study". Glycoconj. J. 11 (6): 507–17. doi:10.1007/bf00731301. PMID 7696853.
- Sharon N, Lis H (November 1990). "Legume lectins--a large family of homologous proteins". FASEB J. 4 (14): 3198–208. doi:10.1096/fasebj.4.14.2227211. PMID 2227211.
- Loris R, Hamelryck T, Bouckaert J, Wyns L (March 1998). "Legume lectin structure". Biochim. Biophys. Acta. 1383 (1): 9–36. doi:10.1016/S0167-4838(97)00182-9. PMID 9546043.
- Manoj N, Suguna K (October 2001). "Signature of quaternary structure in the sequences of legume lectins". Protein Eng. 14 (10): 735–45. doi:10.1093/protein/14.10.735. PMID 11739891.
- PDBe Browser for legume lectin assemblies
- Hamelryck TW, Moore JG, Chrispeels MJ, Loris R, Wyns L (June 2000). "The role of weak protein-protein interactions in multivalent lectin-carbohydrate binding: crystal structure of cross-linked FRIL". J. Mol. Biol. 299 (4): 875–83. doi:10.1006/jmbi.2000.3785. PMID 10843844.