Leucochloridium variae
Leucochloridium variae, the brown-banded broodsac, is a species of trematode whose life cycle involves the alternate parasitic invasion of certain species of snail and bird. While there is no external evidence of the worm's existence within the bird host, the invasion of the snail host involves the grotesque swelling of one or both of the snail's eye stalks as well as the takeover of the snail's brain. This invasion does not cause the snail's death, and snails who are thus invaded sometimes live longer than those which are not.
| Leucochloridium variae | |
|---|---|
.jpg.webp) | |
| Scientific classification | |
| Kingdom: | |
| Phylum: | |
| Class: | |
| Order: | |
| Family: | |
| Genus: | |
| Species: | L. variae |
| Binomial name | |
| Leucochloridium variae McIntosh, 1932 | |
The swollen, pulsating eye stalk resembles a maggot. This modification attracts the parasite's definitive hosts, birds: the bird rips off the eye stalk and eats it, thus becoming infected with the sexually mature parasites. Later on the parasite's eggs are dropped with the bird's feces. Similar life-histories are found in most species in the genus Leucochloridium, including Leucochloridium paradoxum.
The snail regenerates a replacement eye stalk, which can also become reinfected by the parasite.
Life-cycle
The lifecycle of Leucochloridium variae is characterized by the infection of a definitive avian host through the ingestion of sporocysts contained in the intermediate Succinea host. Leucochloridium variae primarily live in the cloaca and intestine of their bird host, while the sporocysts live in the hepatopancreas, haemocoel and the ocular tentacles of Succineidae land snails.[1]
Transmission and infection of intermediate snail hosts
Avian hosts release fluke eggs along with their excreta, which will land on surrounding vegetation for snails to consume. The miracidia will hatch and bore through the snail’s digestive tract. The sporocyst will penetrate and entangle the internal organs of the snail. The sporocysts will continue to grow and multiple by asexual reproduction. The cercariae will develop and encyst in each brood-sac. Brood-sacs will migrate to the snail’s tentacles, where they will mimic the behavior of caterpillars to attract insectivorous birds.[2][3] The growth and reproductive intensity of Leucochloridium variae is regulated at the level of the organism because a single sporocyst can establish a life-cycle within the infected snail. However, snails can be infected by more than one species of Leucochloridium.[4]
Sporocyst description
Snails infected with the sporocyst exhibit distended tentacles, which disrupts the snail’s normal ability to retract into their shell. Brood-sacs may contain multiple free floating metacercariae, which will move about in pulsating manor. Light intensity affects the rate at which the brood sacs pulsate. Brood sacs will normally pulsate between forty and eighty times per minute. The pulsating movement is described as an alternation of shortening and lengthening of the brood sac. Brood sacs will not show movement in complete darkness.[5]
Transmission and infection of avian definitive hosts
The insectivorous birds are attracted to the pulsating of the metacercariae in the sporocyst. This will cause the birds to attack and ingest the brood sacs located in the snail's tentacles. After digestion of the broodsac, sporocysts will become cercaria and further develop into adults. Adult Leucochloridium variae are hermaphroditic helminths, but can cross fertilize with other worms if in close enough proximity. The gravid adults will release their eggs into the intestines of the bird to be excreted out with the bird’s feces; thus, continuing the Leucochloridium lifecycle.[6] Intense infection by the worms can lead emaciation and death in birds. Birds may also freeze to death from the lack of adipose tissue.[7]
Gravid adult description
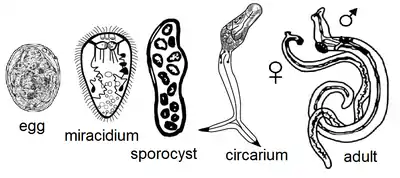
Adult worms are characterized by a flesh-colored body containing an egg-filled uterus that appears black by reflected light. The worms contain a cuticula with a subterminal oral sucker. They also contain a muscular pharynx, ventral sucker, and ceca. The gonads of the worm are arranged in a triangle, containing an ovary with an anterior and posterior testis.[5] The oral sucker is primarily used for the attachment to the avian cloaca. It must withstand the constriction of cloaca, which occurs during defecation. Leucochloridium contains a smooth oral sucker, which functions by forming a tight seal against the host’s mucosa. Leucochloridium also contains a smooth dorsal side, which aids in decreasing friction of passing stool. The rest of the fluke is covered in microvilli that are used to anchor it to the inside of the cloaca. Leucochloridium variae tegument is considered finely spined.[8]
Behavioral differences in infected intermediate hosts
Parasites often induce certain behavioral changes in their hosts in order to aid in the transmission and completion of its life cycle. Land snails parasitized by Leucochloridium spp. will experience phenotypic modification through the pulsating brood sacs. Infected snails were found to have increased mobility, which allows them to migrate to higher and more well lit areas. Healthy snails seek darkness to hide from predators, but the infected amber snail moves itself into dangerous open and well lit spaces. Thus, making them more susceptible and accessible to avian predation.[9][10]
Distribution and history
Different species of Leucochloridium can be found all over the planet, while Leucochloridium variae is specifically found in North America. The first known description of L. variae was written by McIntosh in 1932. L. variae commonly parasitizes Mniotilta varia and has been collected from lakes in the Michigan area.[3][11][12] Other known locations that L. variae are known to inhabit are Iowa,[13] Nebraska,[14][15] Ohio[16] and others.
Hosts
Intermediate host of Leucochloridium variae include:
There was no finding of difference in length of shells in parasitized and in non-parasitized snails.[16]
Hosts of Leucochloridium variae include:
- American robin[18]
- Common gull - experimental host[15]
- Zebra finch - experimental host[15]
References
- Yamada, Seitaro; Fukumoto, Shin-ichiro (August 2011), Isolation of sporocyst broodsacs of the Genus Leucochloridium (Leucochloridiidae: Trematoda) from the intermediate host, Succunea lauta, in Japan, Graduate School of Veterinary Medicine, Hokkaido University, doi:10.14943/jjvr.59.2-3.101
- Woodhead, Arthur E. (1935). "The Mother Sporocysts of Leucochloridium". The Journal of Parasitology. 21 (5): 337–346. doi:10.2307/3271943. ISSN 0022-3395. JSTOR 3271943.
- MCINTOSH, LOIS (1948). "Leucochloridium Sporocysts from the Okoboji Region". scholarworks.
- Ataev, G. L.; Zhukova, A. A.; Tokmakova, А. S.; Prokhorova, Е. E. (August 2016). "Multiple infection of amber Succinea putris snails with sporocysts of Leucochloridium spp. (Trematoda)". Parasitology Research. 115 (8): 3203–3208. doi:10.1007/s00436-016-5082-6. ISSN 0932-0113. PMID 27112757. S2CID 14985813.
- Robinson, Edwin J. (1947). "Notes on the Life History of Leucochloridium fuscostriatum n. sp. provis. (Trematoda: Brachylaemidae)". The Journal of Parasitology. 33 (6): 467–475. doi:10.2307/3273326. ISSN 0022-3395. JSTOR 3273326. PMID 18903602.
- DeLaCruz, David. "Leucochloridium paradoxum". Animal Diversity Web. Retrieved 2020-02-27.
- Okulewicz, A.; Sitko, J. (2012-12-01). "Parasitic helminthes — probable cause of death of birds". Helminthologia. 49 (4): 241–246. doi:10.2478/s11687-012-0045-7. ISSN 1336-9083. S2CID 25123584.
- Bakke, Tor A. (1976). "Functional morphology and surface topography of Leucochloridium sp. (Digenea), revealed by scanning electron microscopy". Zeitschrift für Parasitenkunde. 51 (1): 115–128. doi:10.1007/BF00380533. ISSN 0044-3255. S2CID 34328256.
- Wesołowska, W.; Wesołowski, T. (March 2014). "Do L eucochloridium sporocysts manipulate the behaviour of their snail hosts?: Leucochloridium sporocysts and snail host behaviour". Journal of Zoology. 292 (3): 151–155. doi:10.1111/jzo.12094.
- Staff, ZRS (2013-05-14). "ZOMBIE SNAILS SPREAD INFECTION".
- McIntosh, Allen (1932). "Some New Species of Trematode Worms of the Genus Leucochloridium Carus, Parasitic in Birds from Northern Michigan, with a Key and Notes on Other Species of the Genus". The Journal of Parasitology. 19 (1): 32–53. doi:10.2307/3271429. ISSN 0022-3395. JSTOR 3271429.
- Lewis, Paul D. (1974). "Helminths of Terrestrial Molluscs in Nebraska. II. Life Cycle of Leucochloridium variae McIntosh, 1932 (Digenea: Leucochloridiidae)". The Journal of Parasitology. 60 (2): 251–255. doi:10.2307/3278459. ISSN 0022-3395. JSTOR 3278459. PMID 4821109.
- Bernard Fried, Paul D. Lewis, Jr. and Kelly Beers 1995. Thin-Layer Chromatographic and Histochemical Analyses of Neutral Lipids in the Intramolluscan Stages of Leucochloridium variae (Digenea, Leucochloridiidae) and the Snail Host, Succinea ovalis. Journal of Parasitology, volume 81(1): 112-114.
- Michael A. Barger & John A. Hnida. 2008. Survey of Trematodes from Terrestrial Gastropods and Small Mammals in Southeastern Nebraska, U.S.A. Comparative Parasitology 75(2):308-314. doi:10.1654/4357.1
- Bakke, Tor A. 1982. The Morphology and Taxonomy of Leucochloridium (L.) variae Mclntosh (Digenea, Leucochloridiidae) from the Nearctic as Revealed by Light and Scanning Electron Microscopy. Zoologica Scripta 11(2):87–100 doi:10.1111/j.1463-6409.1982.tb00521.x
- A Burky & Daniel J. Hornbach. 1979 Comparison of carbon and nitrogen content of infected and uninfected snails, Succinea ovalis, and the trematode Leucochloridium variae. Journal of Parasitology 65(3): 371-374
- Fried B., Beers K., Lewis PD Jr. 1993 (February). Lipids in the broodsac of Leucochloridium variae (Digenea, Leucochloridiidae) and its snail host Succinea ovalis. Int. J. Parasitol. 23(1):129-131.
- Parasites of the Robin Archived July 4, 2010, at the Wayback Machine. Accessed 12 February 2009.
External links
- Paul D. Lewis, Jr. - Helminths of Terrestrial Molluscs in Nebraska. II. Life Cycle of Leucochloridium variae McIntosh, 1932 (Digenea: Leucochloridiidae). - The Journal of Parasitology, Vol. 60, No. 2 (Apr., 1974), pp. 251–255
- Video on YouTube from National Geographic
- Video on YouTube