Lysobacter
The genus Lysobacter belongs to the family Xanthomonadaceae within the Gammaproteobacteria and includes at least 46 named species, including: Lysobacter enzymogenes, L. antibioticus, L. gummosus, L. brunescens, L. defluvii, L. niabensis, L. niastensis, L. daejeonensis, L. yangpyeongensis, L. koreensis, L. concretionis, L. spongiicola, and L. capsici.[1][2][3][4][5][6][7][8] Lysobacter spp. were originally grouped with myxobacteria because they shared the distinctive trait of gliding motility, but they uniquely display a number of traits that distinguish them from other taxonomically and ecologically related microbes including high genomic G+C content (typically ranging between 65 and 72%) and the lack of flagella.[2][9] The feature of gliding motility alone has piqued the interest of many, since the role of gliding bacteria in soil ecology is poorly understood. In addition, while a number of different mechanisms have been proposed for gliding motility among a wide range of bacterial species,[10] the genetic mechanism in Lysobacter remains unknown. Members of the Lysobacter group have gained broad interest for production of extracellular enzymes.[11][12][13][14][15][16][17][18][19][20][21][22][23] The group is also regarded as a rich source for production of novel antibiotics, such as β-lactams containing substituted side chains, macrocyclic lactams and macrocyclic peptide or depsipeptide antibiotics like the katanosins.[24][25][26][27][28][29][30][31][32][33][34][35]
| Lysobacter | |
|---|---|
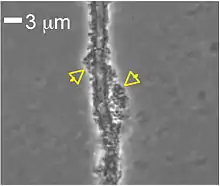 | |
| Attachment of Lysobacter enzymogenes strain C3 to fungal hyphae of Magnaporthe oryzae (also known as rice blast and gray leaf spot of turfgarss) | |
| Scientific classification | |
| Domain: | |
| Phylum: | |
| Class: | |
| Order: | |
| Family: | |
| Genus: | Lysobacter |
Habitat
Lysobacter spp. have been described as ubiquitous inhabitants of soil and water.[2] Their presence has been largely ignored, since members often are minor components in sample screenings when using conventional isolation procedures. However, because of improved molecular methods of identification and better descriptions for the genus, their agricultural relevance is becoming increasingly evident, especially as members of ecologically significant microbial communities associated with soil and plants.[4][9][36][37][38][39][40][41][42] Recent evidence suggests Lysobacter spp. may occupy a wide range of ecological niches beyond those associated with plants, including a broad range of 'extreme' environments. For example, 16S rDNA phylogenetic analyses show Lysobacter clades that include sequences obtained from hydrothermal vents, isolates from Mt. Pinatubo mud flows and upflow anaerobic blanket sludge reactors, and an iron-oxidizing, microaerophilic lithotroph.[1][4][9][43]
Lysobacter gummosus was discovered living on the skin of redback salamanders and producing 2,4-diacetylphloroglucinol, a chemical which inhibits the growth of certain pathogenic fungi.[44]
Biological control
The potential of Lysobacter species as biological control agents for plant diseases has been recognized recently.[9][43] Among L. enzymogenes strains, C3 is the most thoroughly characterized strain at both the molecular and biological levels. The ecological versatility of the strain is reflected by the range of diseases it is able to control, as well as the various plant hosts and plant parts it is capable of colonizing. For example, L. enzymogenes strain C3 (erroneously identified as Stenotrophomonas maltophilia) has been reported to control foliar diseases such as leaf spot of tall fescue caused by Bipolaris sorokiniana,[45] bean rust caused by Uromyces appendiculatus [46] and Fusarium head blight of wheat.[47] L. enzymogenes strain C3 also has been reported to suppress soilborne diseases, such as brown patch in turfgrass caused by Rhizoctonia solani,[48] the seedling disease Pythium damping-off of sugarbeet [49] and summer patch disease of Kentucky bluegrass caused by the root-infecting Magnaporthe poae.[50] Lysobacter sp. SB-K88 has been found to suppress damping-off disease in sugar beet and spinach through antibiosis and characteristic root colonization in perpendicular fashion Islam et al. (2005).
Disease-suppressive soils
Lysobacter species have also been isolated from soils suppressive to Rhizoctonia solani.[51] Clay soils with natural suppressiveness against Rhizoctonia contained higher numbers of antagonistic isolates of L. gummosus, L. antibioticus, and/or L. capsici. Although the mechanism behind this phenomenon is not yet understood, it appeared that growing grass/clover increased the number of these Lysobacter species, as well as the Rhizoctonia suppressiveness.
Mechanisms of antagonism
Originally characterized as a biological control agent for plant diseases, L. enzymogenes strain C3 is unique in that it expresses a wide range of mechanisms contributing to microbial antagonism and biological control that are not shared by all strains of the species. The strain produces numerous extracellular enzymes that contribute to biocontrol activity, including multiple forms of β-1,3-glucanases and chitinases.[19][52] The strain also has been demonstrated to induce systemic resistance in certain plants, protecting them from pathogen infection.[53][54] In addition, recent studies have indicated important roles for secondary metabolites with antibiotic activity and biosurfactant activity in fungal antagonism.[50] Several of these traits are globally controlled by a regulator encoded by the clp gene.[49][50] Mutations in clp are intriguing for two reasons. First, the mutant phenotype implies that a broad range of genes is involved in secreted antimicrobials associated with the clp regulon, many of which remain unidentified. The second is that mutations in clp result in significant loss of extracellular enzyme activities and antimicrobial activity displayed by L. enzymogenes strain C3. These activities normally are phenotypically overwhelming and often lead to masking of other phenotypes in standard assays, making mutation effects of non-related genes difficult or nearly impossible to evaluate. However, strains harboring clp gene mutations provide a means to separate clp-regulated phenotypes from others (such as that describe below), thus making their evaluation feasible. Biological control and mode of actions of disease suppression by Lysobacter spp. has been reviewed Islam 2011.
Lysobacter genetics
L. enzymogenes strain C3 is a genetically tractable strain allowing for easy construction of gene knockouts, supporting its use as a model genetic system for unraveling the molecular basis of pathogenicity, as well as identifying mechanisms of microbial antagonism and biological control. Indeed, a number of derivative strains of L. enzymogenes strain C3 already have been constructed, including mutants affected in structural genes encoding enzyme activities, the regulatory clp gene and various combinations thereof.[19][49]
Species
The genus has 46 known species (July 2018):[55][56]
- Lysobacter aestuarii[57]
- Lysobacter agri[57]
- Lysobacter antibioticus
- Lysobacter arseniciresistens[57]
- Lysobacter brunescens
- Lysobacter burgurensis
- Lysobacter capsici
- Lysobacter caeni[57]
- Lysobacter cavernae[57]
- Lysobacter concretionis
- Lysobacter daejeonensis
- Lysobacter defluvii
- Lysobacter dokdonensis
- Lysobacter enzymogenes
- Lysobacter erysipheiresistens[57]
- Lysobacter firmicutimachus[57]
- Lysobacter fragariae[57]
- Lysobacter ginsengisoli[57]
- Lysobacter gummosus
- Lysobacter hankyongensis[57]
- Lysobacter humi[57]
- Lysobacter koreensis
- Lysobacter korlensis
- Lysobacter lycopersici[57]
- Lysobacter maris[57]
- Lysobacter mobilis[57]
- Lysobacter niabensis
- Lysobacter niastensis
- Lysobacter novalis[57]
- Lysobacter olei[57]
- Lysobacter oligotrophicus[57]
- Lysobacter oryzae
- Lysobacter panacisoli[57]
- Lysobacter panaciterrae
- Lysobacter rhizophilus[57]
- Lysobacter rhizosphaerae[57]
- Lysobacter ruishenii
- Lysobacter sediminicola[57]
- Lysobacter silvestris[57]
- Lysobacter solanacearum[57]
- Lysobacter soli
- Lysobacter spongiicola
- Lysobacter terrae[57]
- Lysobacter terricola[57]
- Lysobacter thermophilus[57]
- Lysobacter tolerans[57]
- Lysobacter ximonensis
- Lysobacter xinjiangensis
- Lysobacter yangpyeongensis
References
- Bae, H. S., W. T. Im, and S. T. Lee. 2005. Lysobacter concretionis sp. nov., isolated from anaerobic granules in an upflow anaerobic sludge blanket reactor. Int J Syst Evol Microbiol 55:1155–61.
- Christensen, P., and F. Cook. 1978. Lysobacter, a new genus of nonfruiting, gliding bacteria with a high base ratio. International Journal of Systematic Bacteriology 28:367–393.
- Lee, J. W., W. T. Im, M. K. Kim, and D. C. Yang. 2006. Lysobacter koreensis sp. nov., isolated from a ginseng field. Int J Syst Evol Microbiol 56:231-5.
- Weon, H. Y., B. Y. Kim, Y. K. Baek, S. H. Yoo, S. W. Kwon, E. Stackebrandt, and S. J. Go. 2006. Two novel species, Lysobacter daejeonensis sp. nov. and Lysobacter yangpyeongensis sp. nov., isolated from Korean greenhouse soils. Int J Syst Evol Microbiol 56:947-51.
- Weon, H. Y., B. Y. Kim, M. K. Kim, S. H. Yoo, S. W. Kwon, S. J. Go, and E. Stackebrandt. 2007. Lysobacter niabensis sp. nov. and Lysobacter niastensis sp. nov., isolated from greenhouse soils in Korea. Int J Syst Evol Microbiol 57:548-51.
- Yassin, A. F., W.-M. Chen, H. Hupfer, C. Siering, R. M. Kroppenstedt, A. B. Arun, W.-A. Lai, F.-T. Shen, P. D. Rekha, and C. C. Young. 2007. Lysobacter defluvii sp. nov., isolated from municipal solid waste. Int J Syst Evol Microbiol 57:1131–1136.
- Romanenko, L.A., Uchino, M., Tanaka, N., Frolova, G.M., Mikhailov, V.V., 2008. Lysobacter spongiicola sp. nov., isolated from a deep-sea sponge. International Journal of Systematic and Evolutionary Microbiology 58, 370–374.
- Park, J.H., Kim, R., Aslam, Z., Jeon, C.O., Chung, Y.R., 2008. Lysobacter capsici sp. nov., with antimicrobial activity, isolated from the rhizosphere of pepper, and emended description of the genus Lysobacter. International Journal of Systematic and Evolutionary Microbiology 58, 387–392.
- Sullivan, R. F., M. A. Holtman, G. J. Zylstra, J. F. White, and D. Y. Kobayashi. 2003. Taxonomic positioning of two biological control agents for plant diseases as Lysobacter enzymogenes based on phylogenetic analysis of 16S rDNA, fatty acid composition and phenotypic characteristics. Journal of Applied Microbiology 94:1079–1086.
- McBride, M. J. 2001. Bacterial gliding motility: Multiple mechanisms for cell movement over surfaces. Annual Review of Microbiology 55:49–75.
- Ahmed, K., S. Chohnan, H. Ohashi, T. Hirata, T. Masaki, and F. Sakiyama. 2003. Purification, bacteriolytic activity, and specificity of β-lytic protease from Lysobacter sp. IB-9374. Journal of Bioscience and Bioengineering 95:27–34.
- Allpress, J. D., G. Mountain, and P. C. Gowland. 2002. Production, purification and characterization of an extracellular keratinase from Lysobacter NCIMB 9497. Lett Appl Microbiol 34:337-42.
- Au, S., K. L. Roy, and R. G. von Tigerstrom. 1991. Nucleotide sequence and characterization of the gene for secreted alkaline phosphatase from Lysobacter enzymogenes. J Bacteriol 173:4551-7.
- Chohnan, S., J. Nonaka, K. Teramoto, K. Taniguchi, Y. Kameda, H. Tamura, Y. Kurusu, S. Norioka, T. Masaki, and F. Sakiyama. 2002. Lysobacter strain with high lysyl endopeptidase production. FEMS Microbiol Lett 213:13–20.
- Chohnan, S., K. Shiraki, K. Yokota, M. Ohshima, N. Kuroiwa, K. Ahmed, T. Masaki, and F. Sakiyama. 2004. A second lysine-specific serine protease from Lysobacter sp. strain IB-9374. J Bacteriol 186:5093-100.
- Epstein, D. M., and P. C. Wensink. 1988. The α-lytic protease gene of Lysobacter enzymogenes. The nucleotide sequence predicts a large prepropeptide with homology to propeptides of other chymotrypsin-like enzymes. J Biol Chem 263:16586-90.
- Ogura, J., A. Toyoda, T. Kurosawa, A. L. Chong, S. Chohnan, and T. Masaki. 2006. Purification, characterization, and gene analysis of cellulase (Cel8A) from Lysobacter sp. IB-9374. Biosci Biotechnol Biochem 70:2420-8.
- Palumbo, J. D., R. F. Sullivan, and D. Y. Kobayashi. 2003. Molecular characterization and expression in Escherichia coli of three β-1,3-Glucanase genes from Lysobacter enzymogenes Strain N4-7. J. Bacteriol. 185:4362–4370.
- Palumbo, J. D., G. Y. Yuen, C. C. Jochum, K. Tatum, and D. Y. Kobayashi. 2005. Mutagenesis of β-1,3-glucanase genes in Lysobacter enzymogenes strain C3 results in reduced biological control activity toward Bipolaris leaf spot of tall fescue and Pythium damping-off of sugar beet. Phytopathology 95:701–707.
- von Tigerstrom, R. G. 1980. Extracellular nucleases of Lysobacter enzymogenes: production of the enzymes and purification and characterization of an endonuclease. Can J Microbiol 26:1029–37.
- von Tigerstrom, R. G. 1984. Production of two phosphatases by Lysobacter enzymogenes and purification and characterization of the extracellular enzyme. Appl Environ Microbiol 47:693-8.
- von Tigerstrom, R. G., and S. Stelmaschuk. 1987. Comparison of the phosphatases of Lysobacter enzymogenes with those of related bacteria. J Gen Microbiol 133:3121-7.
- Wright, D. S., L. D. Graham, and P. A. Jennings. 1998. Cloning of a Lysobacter enzymogenes gene that encodes an arginyl endopeptidase (endoproteinase Arg-C). Biochim Biophys Acta 1443:369-74.
- Bonner, D. P., J. O'Sullivan, S. K. Tanaka, J. M. Clark, and R. R. Whitney. 1988. Lysobactin, a novel antibacterial agent produced by Lysobacter sp. II. Biological properties. J Antibiot (Tokyo) 41:1745–51.
- Harada, S., S. Tsubotani, H. Ono, and H. Okazaki. 1984. Cephabacins, new cephem antibiotics of bacterial origin. II. Isolation and characterization. J Antibiot (Tokyo) 37:1536–45.
- Hashizume, H., S. Hattori, M. Igarashi, and Y. Akamatsu. 2004. Tripropeptin E, a new tripropeptin group antibiotic produced by Lysobacter sp. BMK333-48F3. J Antibiot (Tokyo) 57:394-9.
- Hashizume, H., S. Hirosawa, R. Sawa, Y. Muraoka, D. Ikeda, H. Naganawa, and M. Igarashi. 2004. Tripropeptins, novel antimicrobial agents produced by Lysobacter sp. J Antibiot (Tokyo) 57:52-8.
- Hashizume, H., M. Igarashi, S. Hattori, M. Hori, M. Hamada, and T. Takeuchi. 2001. Tripropeptins, novel antimicrobial agents produced by Lysobacter sp. I. Taxonomy, isolation and biological activities. J Antibiot (Tokyo) 54:1054-9.
- Kato, A., S. Nakaya, N. Kokubo, Y. Aiba, Y. Ohashi, H. Hirata, K. Fujii, and K. Harada. 1998. A new anti-MRSA antibiotic complex, WAP-8294A. I. Taxonomy, isolation and biological activities. J Antibiot (Tokyo) 51:929-35.
- Kimura, H., M. Izawa, and Y. Sumino. 1996. Molecular analysis of the gene cluster involved in cephalosporin biosynthesis from Lysobacter lactamgenus YK90. Applied Microbiology and Biotechnology 44:589–596.
- Meyers, E., R. Cooper, L. Dean, J. H. Johnson, D. S. Slusarchyk, W. H. Trejo, and P. D. Singh. 1985. Catacandins, novel anticandidal antibiotics of bacterial origin. J Antibiot (Tokyo) 38:1642-8.
- Nakayama, T., Y. Homma, Y. Hashidoko, J. Mizutani, and S. Tahara. 1999. Possible role of xanthobaccins produced by Stenotrophomonas sp strain SB-K88 in suppression of sugar beet damping-off disease. Applied and Environmental Microbiology 65:4334–4339.
- O'Sullivan, J., J. E. McCullough, A. A. Tymiak, D. R. Kirsch, W. H. Trejo, and P. A. Principe. 1988. Lysobactin, a novel antibacterial agent produced by Lysobacter sp. I. Taxonomy, isolation and partial characterization. J Antibiot (Tokyo) 41:1740-4.
- Ono, H., Y. Nozaki, N. Katayama, and H. Okazaki. 1984. Cephabacins, new cephem antibiotics of bacterial origin. I. Discovery and taxonomy of the producing organisms and fermentation. J Antibiot (Tokyo) 37:1528–35.
- Panthee, S; Hamamoto, H; Paudel, A; Sekimizu, K (November 2016). "Lysobacter species: a potential source of novel antibiotics". Archives of Microbiology. 198 (9): 839–45. doi:10.1007/s00203-016-1278-5. PMID 27541998.
- Folman, L. B., J. Postma, and J. A. Van Veen. 2001. Ecophysiological characterization of rhizosphere bacterial communities at different root locations and plant developmental stages of cucumber grown on rockwool. Microbial Ecology 42:586–597.
- Islam, M. T., Y. Hashidoko, A. Deora, T. Ito, and S. Tahara. 2005. Suppression of damping-off disease in host plants by the rhizoplane bacterium Lysobacter sp. Strain SB-K88 Is linked to plant colonization and antibiosis against soilborne Peronosporomycetes. Appl. Environ. Microbiol. 71:3786–3796.
- Lee, M. S., J. O. Do, M. S. Park, S. Jung, K. H. Lee, K. S. Bae, S. J. Park, and S. B. Kim. 2006. Dominance of Lysobacter sp. in the rhizosphere of two coastal sand dune plant species, Calystegia soldanella and Elymus mollis. Antonie Van Leeuwenhoek 90:19–27.
- Lueders, T., R. Kindler, A. Miltner, M. W. Friedrich, and M. Kaestner. 2006. Identification of bacterial micropredators distinctively active in a soil microbial food web. Appl. Environ. Microbiol. 72:5342–5348.
- Nour, S. M., J. R. Lawrence, H. Zhu, G. D. W. Swerhone, M. Welsh, T. W. Welacky, and E. Topp. 2003. Bacteria associated with cysts of the soybean cyst nematode (Heterodera glycines). Applied and Environmental Microbiology 69:607–615.
- Roesti, D., K. Ineichen, O. Braissant, D. Redecker, A. Wiemken, and M. Aragno. 2005. Bacteria associated with spores of the arbuscular mycorrhizal fungi Glomus geosporum and Glomus constrictum. Appl Environ Microbiol 71:6673-9.
- Schmalenberger, A., and C. C. Tebbe. 2003. Bacterial diversity in maize rhizospheres: conclusions on the use of genetic profiles based on PCR-amplified partial small subunit rRNA genes in ecological studies. Molecular Ecology 12:251–261.
- Folman, L. B., J. Postma, and J. A. van Veen. 2003. Characterisation of Lysobacter enzymogenes (Christensen and Cook 1978) strain 3.1T8, a powerful antagonist of fungal diseases of cucumber. Microbiological Research 158:107–115.
- Brucker RM, Baylor CM, Walters RL, Lauer A, Harris RN, Minbiole KPC. 2008. The identification of 2,4-diacetylphloroglucinol as an antifungal metabolite produced by cutaneous bacteria of the salamander Plethodon cinereus. Journal of Chemical Ecology 34(1):39–43.
- Zhang, Z., and G. Y. Yuen. 1999. Biological control of Bipolaris sorakiniana on tall fescue by Stenotrophomonas maltophilia strain C3. Phytopathology 89:817–822.
- Yuen, G. Y., J. R. Steadman, D. T. Lindgren, D. Schaff, and C. Jochum. 2001. Bean rust biological control using bacterial agents. Crop Protection 20:395–402.
- Jochum, C. C., L. E. Osborne, and G. Y. Yuen. 2006. Fusarium head blight biological control with Lysobacter enzymogenes. Biological Control 39:336–344.
- Giesler, L. J., and G. Y. Yuen. 1998. Evaluation of Stenotrophomonas maltophilia strain C3 for biocontrol of brown patch disease. Crop Protection 17:509–513.
- Kobayashi, D. Y., R. M. Reedy, J. D. Palumbo, J.-M. Zhou, and G. Y. Yuen. 2005. A clp gene homologue belonging to the crp gene family globally regulates lytic enzyme production, antimicrobial activity, and biological control activity expressed by Lysobacter enzymogenes strain C3. Appl. Environ. Microbiol. 71:261–269.
- Kobayashi, D. Y., and G. Y. Yuen. 2005. The role of clp-regulated factors in antagonism against Magnaporthe poae and biological control of summer patch disease of Kentucky bluegrass by Lysobacter enzymogenes C3. Can J Microbiol 51:719-23.
- Postma, J., Schilder, M.T., Bloem, J., Van Leeuwen-Haagsma, W.K., 2008. Soil suppressiveness and functional diversity of the soil microflora in organic farming systems. Soil Biology and Biochemistry 40, 2394–2406.
- Zhang, Z., G. Y. Yuen, G. Sarath, and A. R. Penheiter. 2001. Chitinases from the plant disease biocontrol agent, Stenotrophomonas maltophilia C3. Phytopathology 91:204–211.
- Kilic-Ekici, O., and G. Y. Yuen. 2004. Comparison of strains of Lysobacter enzymogenes and PGPR for induction of resistance against Bipolaris sorokiniana in tall fescue. Biological Control 30:446–455.
- Kilic-Ekici, O., and G. Y. Yuen. 2003. Induced resistance as a mechanism of biological control by Lysobacter enzymogenes strain C3. Phytopathology 93:1103–1110.
- Opname van Lysobacter in DSMZ
- Lysobacter korlensis sp. nov. and Lysobacter burgurensis sp. nov., isolated from soil. door Lei Zhang e.a. (2011, International Journal of Systematic and Evolutionary Microbiology)
- Parte, A.C. "Lysobacter". LPSN.
