Marrow adipose tissue
Marrow adipose tissue (MAT), also known as bone marrow adipose tissue (BMAT), is a type of fat deposit in bone marrow. It increases in states of low bone density -osteoporosis,[1][2] anorexia nervosa/ caloric restriction,[3][4] skeletal unweighting such as that which occurs in space travel,[5][6] anti-diabetes therapies.[7]
| Marrow adipose tissue | |
|---|---|
_differentiation.png.webp) | |
| Anatomical terminology |
Origin
The marrow adipocytes originate from mesenchymal stem cell (MSC) progenitors that also give rise to osteoblasts, among other cell types.[8] Thus, it is thought that MAT results from preferential MSC differentiation into the adipocyte, rather than osteoblast, lineage in the setting of osteoporosis.[9] Since MAT is increased in the setting of obesity[10][11][12] and is suppressed by endurance exercise,[13][10][14][15] or vibration,[16] it is likely that MAT physiology, in the setting of mechanical input/exercise, approximates that of white adipose tissue (WAT).
Exercise regulation of marrow adipose tissue
The first study to demonstrate exercise regulation of MAT in rodents was published in 2014;[10] Now, exercise regulation of MAT has been confirmed in a humansl[17] adding clinical importance. Several studies demonstrated exercise reduction of MAT which occurs along with an increase in bone quantity.[15][13][14][18] Since exercise increases bone quantity, reduces MAT and increases expression of markers of fatty acid oxidation in bone, MAT is thought to be providing needed fuel for exercise-induced bone formation or anabolism.[14] One notable exception occurs in the setting of caloric restriction: exercise suppression of MAT does not yield an increase in bone formation and even appears to cause bone loss.[4][19][18] Indeed, energy availability appears to be a factor in the ability of exercise to regulate MAT.
Relationships to other types of fat
MAT has qualities of both white and brown fat.[20] Subcutaneous white fat contain excess energy, indicating a clear evolutionary advantage during times of scarcity. WAT is also the source of adipokines and inflammatory markers which have both positive (e.g., adiponectin)[21] and negative[22] effects on metabolic and cardiovascular endpoints. Visceral abdominal fat (VAT) is a distinct type of WAT that is "proportionally associated with negative metabolic and cardiovascular morbidity",[23] regenerates cortisol,[24] and recently has been tied to decreased bone formation[25][26] Both types of WAT substantially differ from brown adipose tissue (BAT) as by a group of proteins that help BAT’s thermogenic role.[27] MAT, by its "specific marrow location, and its adipocyte origin from at least LepR+ marrow MSC is separated from non-bone fat storage by larger expression of bone transcription factors",[28] and likely indicates a different fat phenotype.[29] Recently, MAT was noted to "produce a greater proportion of adiponectin - an adipokine associated with improved metabolism - than WAT",[30] suggesting an endocrine function for this depot, akin, but different, from that of WAT.
Impact on bone health
MAT increases in states of bone fragility. MAT is thought to result from preferential MSC differentiation into an adipocyte, rather than osteoblast lineage in osteoporosis[31][18] based on the inverse relationship between bone and MAT in bone-fragile osteoporotic states. An increase in MAT is noted in osteoporosis clinical studies measured by MR Spectroscopy.[32][33][34] Estrogen therapy in postmenopausal osteoporosis reduces MAT.[35] Antiresorptive therapies like risedronate or zoledronate also decrease MAT while increasing bone density, supporting an inverse relationship between bone quantity and MAT. During aging, bone quantity declines[36][37] and fat redistributes from subcutaneous to ectopic sites such as bone marrow, muscle, and liver.[38] Aging is associated with lower osteogenic and greater adipogenic biasing of MSC.[39] This aging-related biasing of MSC away from osteoblast lineage may represent higher basal PPARγ expression[40] or decreased Wnt10b.[41][42][43] Thus, bone fragility, osteoporosis, and osteoporotic fractures are thought to be linked to mechanisms which promote MAT accumulation.
- Histologic sections demonstrating Marrow Adipocytes
_cropped.jpg.webp) Representative distal femur histologic section of a 16-week old healthy C57BL/6 mouse demonstrating a typical quantity of marrow adipocytes.
Representative distal femur histologic section of a 16-week old healthy C57BL/6 mouse demonstrating a typical quantity of marrow adipocytes.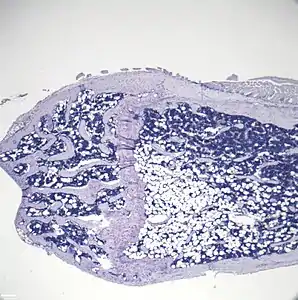 Representative distal femur histologic section of a 16-week old C57BL/6 mouse after 6 weeks of calorie restriction demonstrating an increased quantity of marrow adipocytes.
Representative distal femur histologic section of a 16-week old C57BL/6 mouse after 6 weeks of calorie restriction demonstrating an increased quantity of marrow adipocytes.
Maintenance of hematopoietic stem cells
Bone marrow adipocytes secrete factors that promote HSC renewal in most bones.[44]
Hematopoietic cells (also known as blood cells) reside in the bone marrow along with marrow adipocytes. These hematopoietic cells are derived from hematopoietic stem cells (HSC) which give rise to diverse cells: cells of the blood, immune system, as well as cells that break down bone (osteoclasts). HSC renewal occurs in the marrow stem cell niche, a microenvironment that contains cells and secreted factors that promote appropriate renewal and differentiation of HSC. The study of the stem cell niche is relevant to the field of oncology in order to improve therapy for multiple hematologic cancers. As such cancers are often treated with bone marrow transplantation, there is interest in improving the renewal of HSC.
Measurement
In order to understand the physiology of MAT, various analytic methods have been applied. Marrow adipocytes are difficult to isolate and quantify because they are interspersed with bony and hematopoietic elements. Until recently, qualitative measurements of MAT have relied on bone histology,[45][46] which is subject to site selection bias and cannot adequately quantify the volume of fat in the marrow. Nevertheless, histological techniques and fixation make possible visualization of MAT, quantification of adipocyte size, and MAT’s association with the surrounding endosteum, milieu of cells, and secreted factors.[47][48][49]
Recent advances in cell surface and intracellular marker identification and single-cell analyses led to greater resolution and high-throughput ex-vivo quantification. Flow cytometric quantification can be used to purify adipocytes from the stromal vascular fraction of most fat depots.[50] Early research with such machinery cited adipocytes as too large and fragile for cytometer-based purification, rendering them susceptible to lysis; however, recent advances have been made to mitigate this;[51] nevertheless, this methodology continues to pose technical challenges[52] and is inaccessible to much of the research community.
To improve quantification of MAT, novel imaging techniques have been developed as a means to visualize and quantify MAT. Although proton magnetic resonance spectroscopy (1H-MRS) has been used with success to quantify vertebral MAT in humans,[53] it is difficult to employ in laboratory animals.[54] Magnetic resonance imaging (MRI) provides MAT assessment in the vertebral skeleton[55] in conjunction with μCT-based marrow density measurements.[56] A volumetric method to identify, quantify, and localize MAT in rodent bone has been recently developed, requiring osmium staining of bones and μCT imaging,[57] followed by advanced image analysis of osmium-bound lipid volume (in mm3) relative to bone volume.[10][14][13] This technique provides reproducible quantification and visualization of MAT, enabling the ability to consistently quantify changes in MAT with diet, exercise, and agents that constrain precursor lineage allocation. Although the osmium method is quantitatively precise, osmium is toxic and cannot be compared across batched experiments. Recently, researchers developed and validated[14] a 9.4T MRI scanner technique that allows localization and volumetric (3D) quantification that can be compared across experiments, as in.[4]
- Methods for Quantification of Marrow Adipose Tissue (MAT)
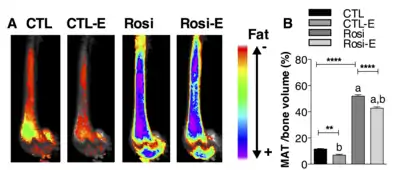 Figure. This figure demonstrates the use of the osmium- μCT method with advanced image processing to quantify MAT. In this figure, running exercise is shown to suppress MAT despite PPARγ agonist. Fat binder osmium is imaged via μCT (A ) in n =5 per group overlaid images. Quantification of osmium as MAT/ bone volume in the whole femur is shown. a, significant due to Rosi. b, significant due to exercise. Rosi=rosiglizaone, CTL=control, E=exercise.
Figure. This figure demonstrates the use of the osmium- μCT method with advanced image processing to quantify MAT. In this figure, running exercise is shown to suppress MAT despite PPARγ agonist. Fat binder osmium is imaged via μCT (A ) in n =5 per group overlaid images. Quantification of osmium as MAT/ bone volume in the whole femur is shown. a, significant due to Rosi. b, significant due to exercise. Rosi=rosiglizaone, CTL=control, E=exercise.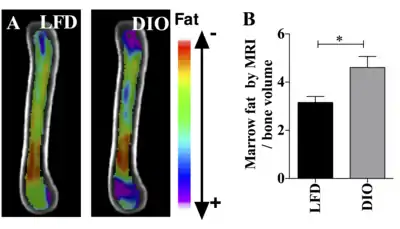 Figure. This demonstrates the use of MRI imaging (9.4T scanner) along with advanced image processing to quantify MAT. The images and graph demonstrate that MAT is higher in obese compared with lean mice. B6 mice were fed HFD from age 4 wk until age 16 wk. MAT was quantified by MRI. A) n=10 superimposed group average images are shown B) MAT normalized to bone volume in each group.
Figure. This demonstrates the use of MRI imaging (9.4T scanner) along with advanced image processing to quantify MAT. The images and graph demonstrate that MAT is higher in obese compared with lean mice. B6 mice were fed HFD from age 4 wk until age 16 wk. MAT was quantified by MRI. A) n=10 superimposed group average images are shown B) MAT normalized to bone volume in each group.
References
![]() This article incorporates text by Gabriel M. Pagnotti and Maya Styner available under the CC BY 4.0 license.
This article incorporates text by Gabriel M. Pagnotti and Maya Styner available under the CC BY 4.0 license.
- Cohen A, Dempster DW, Stein EM, Nickolas TL, Zhou H, McMahon DJ, et al. (August 2012). "Increased marrow adiposity in premenopausal women with idiopathic osteoporosis". The Journal of Clinical Endocrinology and Metabolism. 97 (8): 2782–91. doi:10.1210/jc.2012-1477. PMC 3410269. PMID 22701013.
- Meunier P, Aaron J, Edouard C, Vignon G (October 1971). "Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies". Clinical Orthopaedics and Related Research. 80: 147–54. doi:10.1097/00003086-197110000-00021. PMID 5133320.
- Fazeli PK, Horowitz MC, MacDougald OA, Scheller EL, Rodeheffer MS, Rosen CJ, Klibanski A (March 2013). "Marrow fat and bone--new perspectives". The Journal of Clinical Endocrinology and Metabolism. 98 (3): 935–45. doi:10.1210/jc.2012-3634. PMC 3590487. PMID 23393168.
- McGrath C, Sankaran JS, Misaghian-Xanthos N, Sen B, Xie Z, Styner MA, et al. (January 2020). "Exercise Degrades Bone in Caloric Restriction, Despite Suppression of Marrow Adipose Tissue (MAT)". Journal of Bone and Mineral Research. 35 (1): 106–115. doi:10.1002/jbmr.3872. PMC 6980282. PMID 31509274.
- Ahdjoudj S, Lasmoles F, Holy X, Zerath E, Marie PJ (April 2002). "Transforming growth factor beta2 inhibits adipocyte differentiation induced by skeletal unloading in rat bone marrow stroma". Journal of Bone and Mineral Research. 17 (4): 668–77. doi:10.1359/jbmr.2002.17.4.668. PMID 11918224.
- Wronski TJ, Morey ER (1982-01-01). "Skeletal abnormalities in rats induced by simulated weightlessness". Metabolic Bone Disease & Related Research. 4 (1): 69–75. doi:10.1016/0221-8747(82)90011-X. PMID 7121257.
- Rubin MR, Manavalan JS, Agarwal S, McMahon DJ, Nino A, Fitzpatrick LA, Bilezikian JP (October 2014). "Effects of rosiglitazone vs metformin on circulating osteoclast and osteogenic precursor cells in postmenopausal women with type 2 diabetes mellitus". The Journal of Clinical Endocrinology and Metabolism. 99 (10): E1933-42. doi:10.1210/jc.2013-3666. PMID 24905061.
- Muruganandan S, Roman AA, Sinal CJ (January 2009). "Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: cross talk with the osteoblastogenic program". Cellular and Molecular Life Sciences. 66 (2): 236–53. doi:10.1007/s00018-008-8429-z. PMID 18854943. S2CID 5558912.
- Paccou J, Hardouin P, Cotten A, Penel G, Cortet B (October 2015). "The Role of Bone Marrow Fat in Skeletal Health: Usefulness and Perspectives for Clinicians". The Journal of Clinical Endocrinology and Metabolism. 100 (10): 3613–21. doi:10.1210/jc.2015-2338. PMID 26244490.
- Styner M, Thompson WR, Galior K, Uzer G, Wu X, Kadari S, et al. (July 2014). "Bone marrow fat accumulation accelerated by high fat diet is suppressed by exercise". Bone. 64: 39–46. doi:10.1016/j.bone.2014.03.044. PMC 4041820. PMID 24709686.
- Scheller EL, Khoury B, Moller KL, Wee NK, Khandaker S, Kozloff KM, et al. (2016). "Changes in Skeletal Integrity and Marrow Adiposity during High-Fat Diet and after Weight Loss". Frontiers in Endocrinology. 7: 102. doi:10.3389/fendo.2016.00102. PMC 4961699. PMID 27512386.
- Doucette CR, Horowitz MC, Berry R, MacDougald OA, Anunciado-Koza R, Koza RA, Rosen CJ (September 2015). "A High Fat Diet Increases Bone Marrow Adipose Tissue (MAT) But Does Not Alter Trabecular or Cortical Bone Mass in C57BL/6J Mice". Journal of Cellular Physiology. 230 (9): 2032–7. doi:10.1002/jcp.24954. PMC 4580244. PMID 25663195.
- Styner M, Pagnotti GM, Galior K, Wu X, Thompson WR, Uzer G, et al. (August 2015). "Exercise Regulation of Marrow Fat in the Setting of PPARγ Agonist Treatment in Female C57BL/6 Mice". Endocrinology. 156 (8): 2753–61. doi:10.1210/en.2015-1213. PMC 4511140. PMID 26052898.
- Styner M, Pagnotti GM, McGrath C, Wu X, Sen B, Uzer G, et al. (August 2017). "Exercise Decreases Marrow Adipose Tissue Through ß-Oxidation in Obese Running Mice". Journal of Bone and Mineral Research. 32 (8): 1692–1702. doi:10.1002/jbmr.3159. PMC 5550355. PMID 28436105.
- Pagnotti GM, Styner M, Uzer G, Patel VS, Wright LE, Ness KK, et al. (June 2019). "Combating osteoporosis and obesity with exercise: leveraging cell mechanosensitivity". Nature Reviews. Endocrinology. 15 (6): 339–355. doi:10.1038/s41574-019-0170-1. PMC 6520125. PMID 30814687.
- Luu YK, Pessin JE, Judex S, Rubin J, Rubin CT (April 2009). "Mechanical Signals As a Non-Invasive Means to Influence Mesenchymal Stem Cell Fate, Promoting Bone and Suppressing the Fat Phenotype". BoneKEy Osteovision. 6 (4): 132–149. doi:10.1138/20090371. PMC 3255555. PMID 22241295.
- Belavy DL, Quittner MJ, Ridgers ND, Shiekh A, Rantalainen T, Trudel G (April 2018). "Specific Modulation of Vertebral Marrow Adipose Tissue by Physical Activity". Journal of Bone and Mineral Research. 33 (4): 651–657. doi:10.1002/jbmr.3357. PMID 29336053.
- Little-Letsinger, Sarah E.; Pagnotti, Gabriel M.; McGrath, Cody; Styner, Maya (2020-10-17). "Exercise and Diet: Uncovering Prospective Mediators of Skeletal Fragility in Bone and Marrow Adipose Tissue". Current Osteoporosis Reports. doi:10.1007/s11914-020-00634-y. ISSN 1544-1873.
- Southmayd, Emily A; Williams, Nancy I; Mallinson, Rebecca J; De Souza, Mary Jane (2019-03-21). "Energy Deficiency Suppresses Bone Turnover in Exercising Women With Menstrual Disturbances". The Journal of Clinical Endocrinology & Metabolism. 104 (8): 3131–3145. doi:10.1210/jc.2019-00089. ISSN 0021-972X. PMID 30896746.
- Krings A, Rahman S, Huang S, Lu Y, Czernik PJ, Lecka-Czernik B (February 2012). "Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes". Bone. 50 (2): 546–52. doi:10.1016/j.bone.2011.06.016. PMC 3214232. PMID 21723971.
- Ye R, Scherer PE (April 2013). "Adiponectin, driver or passenger on the road to insulin sensitivity?". Molecular Metabolism. 2 (3): 133–41. doi:10.1016/j.molmet.2013.04.001. PMC 3773837. PMID 24049728.
- Tilg H, Moschen AR (October 2006). "Adipocytokines: mediators linking adipose tissue, inflammation and immunity". Nature Reviews. Immunology. 6 (10): 772–83. doi:10.1038/nri1937. PMID 16998510. S2CID 29865593.
- Wronska A, Kmiec Z (June 2012). "Structural and biochemical characteristics of various white adipose tissue depots". Acta Physiologica. 205 (2): 194–208. doi:10.1111/j.1748-1716.2012.02409.x. PMID 22226221.
- Masuzaki H, Paterson J, Shinyama H, Morton NM, Mullins JJ, Seckl JR, Flier JS (December 2001). "A transgenic model of visceral obesity and the metabolic syndrome". Science. 294 (5549): 2166–70. Bibcode:2001Sci...294.2166M. doi:10.1126/science.1066285. PMID 11739957. S2CID 768303.
- Bredella MA, Lin E, Gerweck AV, Landa MG, Thomas BJ, Torriani M, et al. (November 2012). "Determinants of bone microarchitecture and mechanical properties in obese men". The Journal of Clinical Endocrinology and Metabolism. 97 (11): 4115–22. doi:10.1210/jc.2012-2246. PMC 3485587. PMID 22933540.
- Cohen A, Dempster DW, Recker RR, Lappe JM, Zhou H, Zwahlen A, et al. (June 2013). "Abdominal fat is associated with lower bone formation and inferior bone quality in healthy premenopausal women: a transiliac bone biopsy study". The Journal of Clinical Endocrinology and Metabolism. 98 (6): 2562–72. doi:10.1210/jc.2013-1047. PMC 3667251. PMID 23515452.
- Wu J, Cohen P, Spiegelman BM (February 2013). "Adaptive thermogenesis in adipocytes: is beige the new brown?". Genes & Development. 27 (3): 234–50. doi:10.1101/gad.211649.112. PMC 3576510. PMID 23388824.
- Al-Nbaheen M, Vishnubalaji R, Ali D, Bouslimi A, Al-Jassir F, Megges M, et al. (February 2013). "Human stromal (mesenchymal) stem cells from bone marrow, adipose tissue and skin exhibit differences in molecular phenotype and differentiation potential". Stem Cell Reviews and Reports. 9 (1): 32–43. doi:10.1007/s12015-012-9365-8. PMC 3563956. PMID 22529014.
- Gimble JM, Zvonic S, Floyd ZE, Kassem M, Nuttall ME (May 2006). "Playing with bone and fat". Journal of Cellular Biochemistry. 98 (2): 251–66. doi:10.1002/jcb.20777. PMID 16479589.
- Cawthorn WP, Scheller EL, Learman BS, Parlee SD, Simon BR, Mori H, et al. (August 2014). "Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction". Cell Metabolism. 20 (2): 368–375. doi:10.1016/j.cmet.2014.06.003. PMC 4126847. PMID 24998914.
- Paccou J, Hardouin P, Cotten A, Penel G, Cortet B (October 2015). "The Role of Bone Marrow Fat in Skeletal Health: Usefulness and Perspectives for Clinicians". The Journal of Clinical Endocrinology and Metabolism. 100 (10): 3613–21. doi:10.1210/jc.2015-2338. PMID 26244490.
- Duque G, Li W, Adams M, Xu S, Phipps R (May 2011). "Effects of risedronate on bone marrow adipocytes in postmenopausal women". Osteoporosis International. 22 (5): 1547–53. doi:10.1007/s00198-010-1353-8. PMID 20661545. S2CID 27850362.
- Yeung DK, Griffith JF, Antonio GE, Lee FK, Woo J, Leung PC (August 2005). "Osteoporosis is associated with increased marrow fat content and decreased marrow fat unsaturation: a proton MR spectroscopy study". Journal of Magnetic Resonance Imaging. 22 (2): 279–85. doi:10.1002/jmri.20367. PMID 16028245.
- Li X, Kuo D, Schafer AL, Porzig A, Link TM, Black D, Schwartz AV (April 2011). "Quantification of vertebral bone marrow fat content using 3 Tesla MR spectroscopy: reproducibility, vertebral variation, and applications in osteoporosis". Journal of Magnetic Resonance Imaging. 33 (4): 974–9. doi:10.1002/jmri.22489. PMC 3072841. PMID 21448966.
- Syed FA, Oursler MJ, Hefferanm TE, Peterson JM, Riggs BL, Khosla S (September 2008). "Effects of estrogen therapy on bone marrow adipocytes in postmenopausal osteoporotic women". Osteoporosis International. 19 (9): 1323–30. doi:10.1007/s00198-008-0574-6. PMC 2652842. PMID 18274695.
- Khosla S, Riggs BL, Atkinson EJ, Oberg AL, McDaniel LJ, Holets M, et al. (January 2006). "Effects of sex and age on bone microstructure at the ultradistal radius: a population-based noninvasive in vivo assessment". Journal of Bone and Mineral Research. 21 (1): 124–31. doi:10.1359/jbmr.050916. PMC 1352156. PMID 16355281.
- Glatt V, Canalis E, Stadmeyer L, Bouxsein ML (August 2007). "Age-related changes in trabecular architecture differ in female and male C57BL/6J mice". Journal of Bone and Mineral Research. 22 (8): 1197–207. doi:10.1359/jbmr.070507. PMID 17488199.
- Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H, et al. (October 2010). "Fat tissue, aging, and cellular senescence". Aging Cell. 9 (5): 667–84. doi:10.1111/j.1474-9726.2010.00608.x. PMC 2941545. PMID 20701600.
- Kassem M, Marie PJ (April 2011). "Senescence-associated intrinsic mechanisms of osteoblast dysfunctions". Aging Cell. 10 (2): 191–7. doi:10.1111/j.1474-9726.2011.00669.x. PMID 21210937.
- Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B (December 2004). "Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways". Aging Cell. 3 (6): 379–89. doi:10.1111/j.1474-9728.2004.00127.x. PMC 1850101. PMID 15569355.
- Stevens JR, Miranda-Carboni GA, Singer MA, Brugger SM, Lyons KM, Lane TF (October 2010). "Wnt10b deficiency results in age-dependent loss of bone mass and progressive reduction of mesenchymal progenitor cells". Journal of Bone and Mineral Research. 25 (10): 2138–47. doi:10.1002/jbmr.118. PMC 3153316. PMID 20499361.
- Bennett CN, Ouyang H, Ma YL, Zeng Q, Gerin I, Sousa KM, et al. (December 2007). "Wnt10b increases postnatal bone formation by enhancing osteoblast differentiation". Journal of Bone and Mineral Research. 22 (12): 1924–32. doi:10.1359/jbmr.070810. PMID 17708715.
- Chen Q, Shou P, Zheng C, Jiang M, Cao G, Yang Q, et al. (July 2016). "Fate decision of mesenchymal stem cells: adipocytes or osteoblasts?". Cell Death and Differentiation. 23 (7): 1128–39. doi:10.1038/cdd.2015.168. PMC 4946886. PMID 26868907.
- Zhou BO, Yu H, Yue R, Zhao Z, Rios JJ, Naveiras O, Morrison SJ (August 2017). "Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF". Nature Cell Biology. 19 (8): 891–903. doi:10.1038/ncb3570. PMC 5536858. PMID 28714970.
- Bielohuby M, Matsuura M, Herbach N, Kienzle E, Slawik M, Hoeflich A, Bidlingmaier M (February 2010). "Short-term exposure to low-carbohydrate, high-fat diets induces low bone mineral density and reduces bone formation in rats". Journal of Bone and Mineral Research. 25 (2): 275–84. doi:10.1359/jbmr.090813. PMID 19653818.
- Spatz JM, Ellman R, Cloutier AM, Louis L, van Vliet M, Suva LJ, et al. (April 2013). "Sclerostin antibody inhibits skeletal deterioration due to reduced mechanical loading". Journal of Bone and Mineral Research. 28 (4): 865–74. doi:10.1002/jbmr.1807. PMC 4076162. PMID 23109229.
- Rosen CJ, Ackert-Bicknell CL, Adamo ML, Shultz KL, Rubin J, Donahue LR, et al. (November 2004). "Congenic mice with low serum IGF-I have increased body fat, reduced bone mineral density, and an altered osteoblast differentiation program". Bone. 35 (5): 1046–58. doi:10.1016/j.bone.2004.07.008. PMID 15542029.
- Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ (July 2009). "Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment". Nature. 460 (7252): 259–63. Bibcode:2009Natur.460..259N. doi:10.1038/nature08099. PMC 2831539. PMID 19516257.
- David V, Martin A, Lafage-Proust MH, Malaval L, Peyroche S, Jones DB, et al. (May 2007). "Mechanical loading down-regulates peroxisome proliferator-activated receptor gamma in bone marrow stromal cells and favors osteoblastogenesis at the expense of adipogenesis". Endocrinology. 148 (5): 2553–62. doi:10.1210/en.2006-1704. PMID 17317771.
- Majka SM, Miller HL, Sullivan T, Erickson PF, Kong R, Weiser-Evans M, et al. (October 2012). "Adipose lineage specification of bone marrow-derived myeloid cells". Adipocyte. 1 (4): 215–229. doi:10.4161/adip.21496. PMC 3609111. PMID 23700536.
- Majka SM, Miller HL, Helm KM, Acosta AS, Childs CR, Kong R, Klemm DJ (2014). "Analysis and isolation of adipocytes by flow cytometry". Methods of Adipose Tissue Biology, Part A. Methods in Enzymology. 537. pp. 281–96. doi:10.1016/b978-0-12-411619-1.00015-x. ISBN 9780124116191. PMC 4143162. PMID 24480352.
- Bernstein RL, Hyun WC, Davis JH, Fulwyler MJ, Pershadsingh HA (July 1989). "Flow cytometric analysis of mature adipocytes". Cytometry. 10 (4): 469–74. doi:10.1002/cyto.990100416. PMID 2766892.
- Bredella MA, Torriani M, Ghomi RH, Thomas BJ, Brick DJ, Gerweck AV, et al. (January 2011). "Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 in obese women". Obesity. 19 (1): 49–53. doi:10.1038/oby.2010.106. PMC 3593350. PMID 20467419.
- de Paula FJ, Dick-de-Paula I, Bornstein S, Rostama B, Le P, Lotinun S, et al. (September 2011). "VDR haploinsufficiency impacts body composition and skeletal acquisition in a gender-specific manner". Calcified Tissue International. 89 (3): 179–91. doi:10.1007/s00223-011-9505-1. PMC 3157554. PMID 21637996.
- Fazeli PK, Bredella MA, Freedman L, Thomas BJ, Breggia A, Meenaghan E, et al. (September 2012). "Marrow fat and preadipocyte factor-1 levels decrease with recovery in women with anorexia nervosa". Journal of Bone and Mineral Research. 27 (9): 1864–71. doi:10.1002/jbmr.1640. PMC 3415584. PMID 22508185.
- Rantalainen T, Nikander R, Heinonen A, Cervinka T, Sievänen H, Daly RM (May 2013). "Differential effects of exercise on tibial shaft marrow density in young female athletes". The Journal of Clinical Endocrinology and Metabolism. 98 (5): 2037–44. doi:10.1210/jc.2012-3748. PMID 23616150.
- Scheller EL, Troiano N, Vanhoutan JN, Bouxsein MA, Fretz JA, Xi Y, et al. (2014). "Use of osmium tetroxide staining with microcomputerized tomography to visualize and quantify bone marrow adipose tissue in vivo". Methods of Adipose Tissue Biology, Part A. Methods in Enzymology. 537. pp. 123–39. doi:10.1016/b978-0-12-411619-1.00007-0. ISBN 9780124116191. PMC 4097010. PMID 24480344.
Further reading
- "Bone marrow fat tissue secretes hormone that helps body stay healthy". University of Michigan. 3 July 2014. Archived from the original on 15 March 2015.
- "Another reason to exercise: Burning bone fat a key to better bone health". Science Daily. 18 May 2017.
