McN5652
McN5652[1] is a molecule that can be radiolabeled and then used as a radioligand in positron emission tomography (PET) studies. The [11C]-(+)-McN5652 enantiomer binds to the serotonin transporter.[2] The radioligand is used for molecular neuroimaging and for imaging of the lungs.[3]
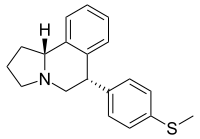 | |
| Names | |
|---|---|
| IUPAC name
rel-(6R,10bS)-6-[4-(Methylsulfanyl)phenyl]-1,2,3,5,6,10b-hexahydropyrrolo[2,1-a]isoquinoline | |
| Other names
trans-McN-5652 | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C19H21NS | |
| Molar mass | 295.44 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
It was developed by Johnson & Johnson's McNeil Laboratories. According to McNeil, McN5652 was among the strongest SRI ever reported at the time of its discovery (sub nM Ki). However, it is not completely 5-HT selective: the racemate has 5-HT=0.68, NA=2.9, and D=36.8nM, whereas (+)-enantiomer has 5-HT=0.39, NA=1.8, and D=23.5 nM. Paroxetine was listed as 5-HT=0.44 nM, NA=20, and DA=460nM in the same paper by the same authors.
Derivatives
McN5652 and related structures have been analyzed for QSAR in terms of binding to the MAT receptor binding site.[4] .]]
See also
- DASB
- JNJ-7925476 (p-ethynyl)
References
- US 4595688 Certain Hexahydro-6-Arylprylpyrrolo [2,1-A]Isoquinoline
- M. Suehiro; U. Scheffel; H. T. Ravert; R. F. Dannals; H. N. Jr Wagner (1993). "[11C](+)McN5652 as a radiotracer for imaging serotonin uptake sites with PET". Life Sciences. 53 (11): 883–92. doi:10.1016/0024-3205(93)90440-E. PMID 8366755.
- Akihiro Takano; Hiroshi Ito; Yasuhiko Sudo; Makoto Inoue; Tetsuya Ichimiya; Fumihiko Yasuno; Kazutoshi Suzuki; Tetsuya Suhara (August 2007). "Effects of smoking on the lung accumulation of [11C]McN5652". Annals of Nuclear Medicine. 21 (6): 349–54. doi:10.1007/s12149-007-0031-1. PMID 17705015.
- Liu, Shuang; Zha, Congxiang; Nacro, Kassoum; Hu, Min; Cui, Wenge; Yang, Yuh-Lin; Bhatt, Ulhas; Sambandam, Aruna; Isherwood, Matthew; Yet, Larry; Herr, Michael T.; Ebeltoft, Sarah; Hassler, Carla; Fleming, Linda; Pechulis, Anthony D.; Payen-Fornicola, Anne; Holman, Nicholas; Milanowski, Dennis; Cotterill, Ian; Mozhaev, Vadim; Khmelnitsky, Yuri; Guzzo, Peter R.; Sargent, Bruce J.; Molino, Bruce F.; Olson, Richard; King, Dalton; Lelas, Snjezana; Li, Yu-Wen; Johnson, Kim; Molski, Thaddeus; Orie, Anitra; Ng, Alicia; Haskell, Roy; Clarke, Wendy; Bertekap, Robert; O’Connell, Jonathan; Lodge, Nicholas; Sinz, Michael; Adams, Stephen; Zaczek, Robert; Macor, John E. (2014). "Design and Synthesis of 4-Heteroaryl 1,2,3,4-Tetrahydroisoquinolines as Triple Reuptake Inhibitors". ACS Medicinal Chemistry Letters. 5 (7): 760–765. doi:10.1021/ml500053b. ISSN 1948-5875. PMC 4094255.
