Mellite
Mellite, also called honeystone, is an unusual mineral being also an organic chemical. It is chemically identified as an aluminium salt of mellitic acid, and specifically as aluminium benzene hexacarboxylate hydrate, with the chemical formula Al2C6(COO)6·16H2O.[3]
| Mellite | |
|---|---|
 | |
| General | |
| Category | Organic minerals |
| Formula (repeating unit) | Al2[C6(COO)6]·16H2O |
| Strunz classification | 10.AC.05 |
| Crystal system | Tetragonal |
| Crystal class | Ditetragonal dipyramidal (4/mmm) H-M symbol: (4/m 2/m 2/m) |
| Space group | I41/acd |
| Unit cell | a = 15.53 Å, c = 23.19 Å; Z = 8 |
| Identification | |
| Color | Honey-yellow, deep red, pale shades of red, brown, gray, white; |
| Crystal habit | Elongated bipyramidal prismatic; as nodules and coatings, fine-grained massive |
| Cleavage | poor/indistinct on {023} |
| Fracture | conchoidal |
| Tenacity | Slightly sectile |
| Mohs scale hardness | 2-2 1⁄2 |
| Luster | Vitreous, resinous, greasy |
| Streak | White |
| Diaphaneity | Transparent to translucent |
| Specific gravity | 1.64 |
| Optical properties | Uniaxial (-) may be anomalously biaxial |
| Refractive index | nω = 1.539 nε = 1.511 |
| Birefringence | δ = 0.028 |
| Pleochroism | Weak; O = yellowish brown; E = yellow |
| Ultraviolet fluorescence | Pale yellow to blue (LW & SW UV) |
| Other characteristics | Pyroelectric |
| References | [1][2][3] |
It is a translucent honey-coloured crystal which can be polished and faceted to form striking gemstones. It crystallizes in the tetragonal system and occurs both in good crystals and as formless masses. It is soft with a Mohs hardness of 2 to 2.5 and has a low specific gravity of 1.6.[1][3]
It was discovered originally in 1789 at Artern in Thuringia, Germany. It has subsequently also been found in Russia, Austria, the Czech Republic, and Hungary. It was named from the Greek μέλι meli "honey",[4] in allusion to its color.[2]
It is found associated with lignite and is assumed to be formed from plant material with aluminium derived from clay.[1]
Structure
The crystal structure of mellite has been determined by neutron diffraction and consists of slightly distorted Al(H2O)63+ octahedra linked by hydrogen bonds to [C6(COO)6]6− mellitate anions and water of crystallization.[5]
 Ball-and-stick model of the asymmetric unit of mellite
Ball-and-stick model of the asymmetric unit of mellite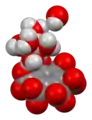 space-filling model of the asymmetric unit
space-filling model of the asymmetric unit Packing of 3×3×3 unit cells
Packing of 3×3×3 unit cells
See also
References
| Wikimedia Commons has media related to Mellite. |
- http://rruff.geo.arizona.edu/doclib/hom/mellite.pdf Handbook of Mineralogy
- http://www.mindat.org/min-2638.html
- http://webmineral.com/data/Mellite.shtml Webmineral data
- μέλι. Liddell, Henry George; Scott, Robert; A Greek–English Lexicon at the Perseus Project.
- Robl, Christian; Kuhs, Werner F. (1991). "A neutron diffraction study on hydrogen bonding in the mineral mellite (Al2[C6(COO)6] · 16H2O) at 15 K". J. Solid State Chem. 92 (1): 101–109. doi:10.1016/0022-4596(91)90246-E.