Mesoxalic acid
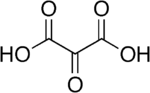
Mesoxalic acid, also called oxomalonic acid or ketomalonic acid, is an organic compound with formula C3H2O5 or HO−(C=O)3−OH.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
oxopropanedioic acid | |
| Other names
Ketomalonic acid Oxomalonic acid α-Ketomalonic acid 2-Oxopropanedioic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.006.796 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3H2O5 | |
| Molar mass | 118.045 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Mesoxalic acid is both a dicarboxylic acid and a ketonic acid. It readily loses two protons to yield the divalent anion C
3O2−
5, called mesoxalate, oxomalonate, or ketomalonate. These terms are also used for salts containing this anion, such as sodium mesoxalate, Na2C3O5; and for esters containing the −C3O5− or −O−(C=O)3−O− moiety, such as diethyl mesoxalate, (C2H5)2C3O5. Mesoxalate is one of the oxocarbon anions, which (like carbonate CO2−
3 and oxalate C
2O2−
4) consist solely of carbon and oxygen.
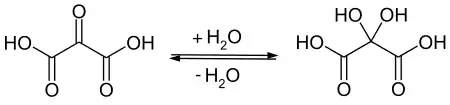
Mesoxalic acid readily absorbs and reacts with water to form a product commonly called "mesoxalic acid monohydrate", more properly dihydroxymalonic acid, HO−(C=O)−C(OH)2−(C=O)−OH.[2] In product catalogs and other contexts, the terms "mesoxalic acid", "oxomalonic acid" and so on often refer to this "hydrated" compound. In particular, the product traded as "sodium mesoxalate monohydrate" is almost always sodium dihydroxymalonate.
Synthesis
Mesoxalic acid can be obtained synthetically by hydrolysis of alloxan with baryta water,[2] by warming caffuric acid[3] with lead acetate solution,[2] or from glycerin diacetate and concentrated nitric acid in the cold. The product can be obtained also by oxidation of tartronic acid[4] or glycerol.[5] Since they are carried out in water, these procedures generally give the dihydroxy derivative.
It is also prepared by the oxidation of glycerol with the help of bismuth(III) nitrate.
See also
References
- Merck Index (12th ed.). p. 5971.
- Henry Enfield Roscoe (1888), A Treatise on Chemistry, volume 3, part2 Organic Chemistry, p. 161. D. Appleton and Co., New York.
- The chemical structure of caffuric acid was given in Allen, W. F. (1932). The preparation and pyrolytic molecular rearrangment [sic] of the 8-ethers of caffeine: And their conversion to 8-methyl and 8-ethylcaffeine. Ann Arbor, Mich.: Edwards Brothers.
- Rosaria Ciriminna and Mario Pagliaro (2004), Oxidation of tartronic acid and dihydroxyacetone to sodium mesoxalate mediated by TEMPO. Tetrahedron Letters, volume 45, issue 34, pp. 6381–6383. doi:10.1016/j.tetlet.2004.07.021
- Rosaria Ciriminna and Mario Pagliaro (2003), One-Pot Homogeneous and Heterogeneous Oxidation of Glycerol to Ketomalonic Acid Mediated by TEMPO. Advanced Synthesis & Catalysis, volume 345, issue 3, Pages 383–388. doi:10.1002/adsc.200390043
