Metaphosphate
A metaphosphate ion is an oxyanion that has the empirical formula PO−
3.[1] The structure of a metaphosphate ion can be described as being made up of PO4 structural units in which each unit shares two corners with another unit. This can come about in two ways.
- Formation of a ring, as in trimetaphosphate, illustrated.
- Formation of an infinite chain, with the same structure as in ammonium metavanadate
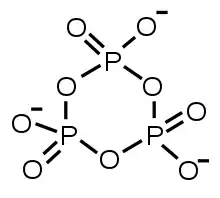
cyclic trimetaphosphate
Metaphosphates can be considered as salts of the corresponding metaphosphoric acids (HnPnO3n) although none of these acids has been isolated. The metaphosphoric acids can be formulated as H2O·P2O5. In comparison, phosphoric acid, H3PO4 can be formulated as 3H2O·P2O5 and pyrophosphoric acid, H4P2O7, as 2H2O·P2O5.[2]
Metaphosphates can be used as an alternative of white phosphorus in organic syntheses.[3]
References
- Averbuch-Pouchot, M.T; Durif, A. (1996). Topics in Phosphate Chemistry. World Scientific Pub Co Inc. ISBN 981-02-2634-9.
- Corbridge, D. (1995). "Chapter 3: Phosphates". Studies in inorganic Chemistry vol. 20. Elsevier Science B.V. pp. 169–305. ISBN 0-444-89307-5.
- "Sidestepping white phosphorus". C&EN Global Enterprise. 96 (7): 7. 2018. doi:10.1021/cen-09607-scicon1. ISSN 2474-7408.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.