Pyrophosphoric acid
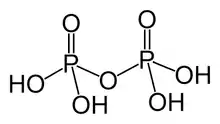
Pyrophosphoric acid, also known as diphosphoric acid, is the inorganic compound with the formula H4P2O7 or, more descriptively, [(HO)2P(O)]2O. Colorless and odorless, it is soluble in water, diethyl ether, and ethyl alcohol. The anhydrous acid crystallizes in two polymorphs, which melt at 54.3 °C and 71.5 °C. The compound is not particularly useful, except that it is a component of polyphosphoric acid and the conjugate acid of the pyrophosphate anion. Anions, salts, and esters of pyrophosphoric acid are called pyrophosphates.
 | |
 | |
| Names | |
|---|---|
| IUPAC names
Diphosphoric acid μ-oxido-bis(dihydroxidooxidophosphorus) | |
| Other names
Diphosphoric acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.017.795 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| H4P2O7 | |
| Molar mass | 177.97 g/mol |
| Melting point | 71.5 °C (160.7 °F; 344.6 K) |
| Extremely soluble | |
| Solubility | Very soluble in alcohol, ether |
| Conjugate base | Pyrophosphate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
It is best prepared by ion exchange from sodium pyrophosphate or by treating lead pyrophosphate with hydrogen sulfide. It is not prepared by dehydration of phosphoric acid. Instead, pyrophosphoric acid is produced as only one of the products.
Reactions
When molten, pyrophosphoric acid rapidly forms an equilibrium mixture of phosphoric acid, pyrophosphoric acid and polyphosphoric acids. The percentage by weight of pyrophosphoric acid is around 40% and it is difficult to recrystallise from the melt. In aqueous solution pyrophosphoric acid, like all polyphosphoric acids, hydrolyses and eventually an equilibrium is established between phosphoric acid, pyrophosphoric acid, and polyphosphoric acids.[1]
- H4P2O7 + H2O ⇌ 2H3PO4
Safety
While pyrophosporic acid is corrosive, it is not known to be otherwise toxic.[2]
History
The name pyrophosphoric acid was given by a "Mr. Clarke of Glasgow" in 1827 who is credited with its discovery following the heating to red heat of a sodium phosphate salt. It was found that phosphoric acid when heated to red heat formed pyrophosphoric acid that was readily converted to phosphoric acid by hot water.[3]
See also
References
- Corbridge, D. (1995). "Chapter 3: Phosphates". Studies in inorganic Chemistry vol. 20. Elsevier Science B.V. pp. 169–305. doi:10.1016/B978-0-444-89307-9.50008-8. ISBN 0-444-89307-5.
- Material Safety Data Sheet: Pyrophosphoric acid MSDS www.sciencelab.com
- Beck, Lewis Caleb (1834). A Manual of Chemistry: Containing a Condensed View of the Present State of the Science, with Copious References to More Extensive Treatises, Original Papers, Etc. E.W & C Skinner. p. 160. Retrieved January 30, 2015.