Methyl hexanoate
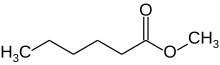
Methyl hexanoate is the fatty acid methyl ester of hexanoic acid (caproic acid), a colourless liquid organic compound with the chemical formula CH
3−(CH
2)
4−COO−CH
3. It is found naturally in many foods and has a role as a plant metabolite. It can also be found in the cytoplasm of cells.[1]
 | |
| Names | |
|---|---|
| IUPAC name
Methyl hexanoate | |
| Systematic IUPAC name
Methyl hexanoate | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| 1744683 | |
| ChEBI | |
| ECHA InfoCard | 100.003.115 |
| EC Number |
|
PubChem CID |
|
| UNII | |
| UN number | 1993 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties[1] | |
| C7H14O2 | |
| Molar mass | 130.187 g·mol−1 |
| Density | 0.8846 |
| Melting point | −71.0 °C (−95.8 °F; 202.2 K) |
| Boiling point | 149.5 °C (301.1 °F; 422.6 K) |
| 1.33 mg/mL at 20 °C | |
| Solubility | ethanol |
Refractive index (nD) |
1.4049 |
| Related compounds | |
Related compounds |
Ethyl hexanoate, Propyl hexanoate, Butyl hexanoate |
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Warning |
| H226 | |
| P210, P233, P240, P241, P242, P243, P280, P303+361+353, P370+378, P403+235, P501 | |
| Flash point | 73 °C; 163 °F; 346 K |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Methyl hexanoate is produced industrially for use as a flavouring agent.[2][3] It can also be used as perfume for a pineapple smell.[4]
Production
Methyl hexanoate is produced in multi-tonne quantities for use as a flavouring agent.[3] It is made by combining Methanol with Hexanoic acid . Naturally,plants metabolize a chemicalto methyl hexanoate.
Uses
Methyl hexanoate is found naturally in foods like potatoes, tomatoes and cheese and is a constituent of some alcoholic beverages.[2] It can be used to mimic the flavor of pineapple like its related ester ethyl hexanoate.[4]
Safety
The LD50 for rats is more than 5 g/kg,[1] indicating low toxicity. When heated to decomposition, methyl hexanoate emits toxic fumes. It can cause burns.
Flammability
Methyl hexanoate is flammable. It has a flash point of 163 °F (73 °C).[1]
See also
References
- "Methyl hexanoate". PubChem. Retrieved 12 August 2020.
- Maarse, Henk (29 March 1991). Volatile Compounds in Foods and Beverages. ISBN 978-0824783907.
- "Methyl hexanoate – Substance Information". European Chemicals Agency. Retrieved 12 August 2020.
- "Methyl hexanoate". The Good Scents Company. Retrieved 15 August 2020.