Mozingo reduction
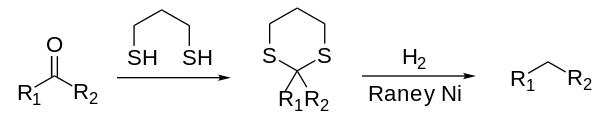
The Mozingo reduction, also known as Mozingo reaction or thioketal reduction, is a chemical reaction capable of fully reducing a ketone or aldehyde to the corresponding alkane.[1][2] The reaction scheme is as follows:[3]
| Mozingo reduction | |
|---|---|
| Named after | Ralph Mozingo |
| Reaction type | Organic redox reaction |
The ketone or aldehyde is activated by conversion to dithioacetal by reaction with a dithiol (nucleophilic substitution). The dithioacetal structure is then hydrogenolyzed using Raney nickel. Raney nickel is converted irreversibly to nickel sulfide. This method is milder than either the Clemmensen or Wolff-Kishner reductions, which employ strongly acidic or basic conditions, respectively, that might interfere with other functional groups.[4]
References
- Francis A. Carey; Richard J. Sundberg (2007). Advanced Organic Chemistry: Reactions and synthesis. Springer. pp. 452–454. ISBN 9780387683508.
- Mosettig, E. and Mozingo, R. 2011. The Rosenmund Reduction of Acid Chlorides to Aldehydes. Organic Reactions. 4:7:362–377. doi:10.1002/0471264180.or004.07
- Jonathan Clayden; Nick Greeves; Stuart Warren (2012). Organic Chemistry (2 ed.). Oxford University Press. ISBN 9780199270293.
- Mithcell, Reginald; Lai, Yee-Hing (1980). "The neutral deoxygenation (reduction) of aryl carbonyl compounds with raney-nickel. an alternative to the clemmenson, wolf-kishner or mozingo (thioketal) reductions". Tetrahedron Letters. Elsevier. 21 (27): 2637–2638. doi:10.1016/S0040-4039(00)92825-9.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
