Multiplex ligation-dependent probe amplification
Multiplex ligation-dependent probe amplification (MLPA) is a variation of the multiplex polymerase chain reaction that permits amplification of multiple targets with only a single primer pair.[1] It detects copy number changes at the molecular level, and software programs are used for analysis. Identification of deletions or duplications can indicate pathogenic mutations, thus MLPA is an important diagnostic tool used in clinical pathology laboratories worldwide.
Description

Each probe consists of two oligonucleotides which recognize adjacent target sites on the DNA. One probe oligonucleotide contains the sequence recognized by the forward primer, the other contains the sequence recognized by the reverse primer. Only when both probe oligonucleotides are hybridized to their respective targets, can they be ligated into a complete probe. The advantage of splitting the probe into two parts is that only the ligated oligonucleotides, but not the unbound probe oligonucleotides, are amplified. If the probes were not split in this way, the primer sequences at either end would cause the probes to be amplified regardless of their hybridization to the template DNA, and the amplification product would not be dependent on the number of target sites present in the sample DNA. Each complete probe has a unique length, so that its resulting amplicons can be separated and identified by (capillary) electrophoresis. This avoids the resolution limitations of multiplex PCR. Because the forward primer used for probe amplification is fluorescently labeled, each amplicon generates a fluorescent peak which can be detected by a capillary sequencer. Comparing the peak pattern obtained on a given sample with that obtained on various reference samples, the relative quantity of each amplicon can be determined. This ratio is a measure for the ratio in which the target sequence is present in the sample DNA.
Various techniques including DGGE (Denaturing Gradient Gel Electrophoresis), DHPLC (Denaturing High Performance Liquid Chromatography), and SSCA (Single Strand Conformation Analysis) effectively identify SNPs and small insertions and deletions. MLPA, however, is one of the only accurate, time-efficient techniques to detect genomic deletions and insertions (one or more entire exons), which are frequent causes of cancers such as hereditary non-polyposis colorectal cancer (HNPCC), breast, and ovarian cancer. MLPA can successfully and easily determine the relative copy number of all exons within a gene simultaneously with high sensitivity.
Relative ploidy
An important use of MLPA is to determine relative ploidy. For example, probes may be designed to target various regions of chromosome 21 of a human cell. The signal strengths of the probes are compared with those obtained from a reference DNA sample known to have two copies of the chromosome. If an extra copy is present in the test sample, the signals are expected to be 1.5 times the intensities of the respective probes from the reference. If only one copy is present the proportion is expected to be 0.5. If the sample has two copies, the relative probe strengths are expected to be equal.
Dosage quotient analysis
Dosage quotient analysis is the usual method of interpreting MLPA data.[2] If a and b are the signals from two amplicons in the patient sample, and A and B are the corresponding amplicons in the experimental control, then the dosage quotient DQ = (a/b) / (A/B). Although dosage quotients may be calculated for any pair of amplicons, it is usually the case that one of the pair is an internal reference probe.
Applications
MLPA facilitates the amplification and detection of multiple targets with a single primer pair. In a standard multiplex PCR reaction, each fragment needs a unique amplifying primer pair. These primers being present in a large quantity result in various problems such as dimerization and false priming. With MLPA, amplification of probes can be achieved. Thus, many sequences (up to 40) can be amplified and quantified using just a single primer pair. MLPA reaction is fast, inexpensive and very simple to perform.
MLPA has a variety of applications[3] including detection of mutations and single nucleotide polymorphisms,[4] analysis of DNA methylation,[5] relative mRNA quantification,[6] chromosomal characterisation of cell lines and tissue samples,[7] detection of gene copy number,[8] detection of duplications and deletions in human cancer predisposition genes such as BRCA1, BRCA2, hMLH1 and hMSH2[9] and aneuploidy determination.[10] MLPA has potential application in prenatal diagnosis both invasive[11] and noninvasive[12]
Recent studies have shown that MLPA (as well as another variants such as iMLPA) is a robust technique for inversion characterisation.[13]
Variants
iMLPA
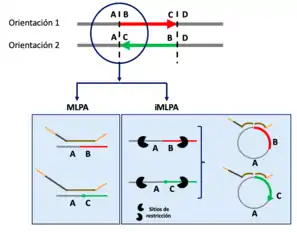
Giner-Delgado, Carla, et al. described a variant of MLPA combining it with iPCR. They call these new method iMLPA[13] and its procedure is the same as MLPA but there are necessary two additional steps at the beginning:
- First, a DNA treatment with restriction enzymes that cut on both sides of the region of interest is necessary.
- The fragments obtained from digestion are recircularized and linked
The probe design is quite similar. Each probe will be formed by two parts that have at least: a target sequence, which is a region that contains the sequence complementary to the region of interest, so that the correct hybridization can occur. And a primer sequence at the end, it is a sequence whose design varies and is what will allow the design of primers and subsequent fragment amplification. In addition, one of the parts of the probe usually contains a stuffer between the target sequence and the primer sequence. The use of different stuffers allows the identification of probes with the same primer sequences but different target sequences, that is key for multiple amplification of several different fragments in a single reaction.
The next step continues with the typical MLPA protocol.[1]
References
- Schouten JP, McElgunn CJ, Waaijer R, Zwijnenburg D, Diepvens F, Pals G (2002). "Relative quantification of 40 nucleic acid sequences by multiplex ligation-dependent probe amplification". Nucleic Acids Res. 30 (12): 57e–57. doi:10.1093/nar/gnf056. PMC 117299. PMID 12060695.
- Yau SC, Bobrow M, Mathew CG, Abbs SJ (1996). "Accurate diagnosis of carriers of deletions and duplications in Duchenne/Becker muscular dystrophy by fluorescent dosage analysis". J. Med. Genet. 33 (7): 550–558. doi:10.1136/jmg.33.7.550. PMC 1050661. PMID 8818939.
- List of MLPA related articles Archived 2007-02-20 at the Wayback Machine
- Volikos E, Robinson J, Aittomaki K, Mecklin JP, Jarvinen H, Westerman AM, de Rooji FW, Vogel T, Moeslein G, Launonen V, Tomlinson IP, Silver AR, Aaltonen LA (2006). "LKB1 exonic and whole gene deletions are a common cause of Peutz‐Jeghers syndrome". J. Med. Genet. 43 (5): e18. doi:10.1136/jmg.2005.039875. PMC 2564523. PMID 16648371.
- Procter M, Chou LS, Tang W, Jama M, Mao R (2006). "Molecular diagnosis of Prader-Willi and Angelman syndromes by methylation-specific melting analysis and methylation-specific multiplex ligation-dependent probe amplification" (PDF). Clin. Chem. 52 (7): 1276–1283. doi:10.1373/clinchem.2006.067603. PMID 16690734.
- Wehner M, Mangold E, Sengteller M, Friedrichs N, Aretz S, Friedl W, Propping P, Pagenstecher C (2005). "Hereditary nonpolyposis colorectal cancer: pitfalls in deletion screening in MSH2 and MLH1 genes". Eur. J. Hum. Genet. 13 (8): 983–986. doi:10.1038/sj.ejhg.5201421. PMID 15870828.
- Wilting SM, Snijders PJ, Meijer GA, Ylstra B, Van den IJssel PR, Snijders AM, Albertson DG, Coffa J, Schouten JP, van de Wiel MA, Meijer CJ, Steenbergen RD (2006). "Increased gene copy numbers at chromosome 20q are frequent in both squamous cell carcinomas and adenocarcinomas of the cervix". J. Pathol. 209 (2): 220–230. doi:10.1002/path.1966. PMID 16538612.
- Introduction to MLPA
- Bunyan DJ, Eccles DM, Sillibourne J, Wilkins E, Thomas NS, Shea-Simonds J, Duncan PJ, Curtis CE, Robinson DO, Harvey JF, Cross NC (2004). "Dosage analysis of cancer predisposition genes by multiplex ligation-dependent probe amplification". Br. J. Cancer. 91 (6): 1155–1159. doi:10.1038/sj.bjc.6602121. PMC 2747696. PMID 15475941.
- Gerdes T, Kirchhoff M, Lind AM, Larsen GV, Schwartz M, Lundsteen C (2005). "Computer-assisted prenatal aneuploidy screening for chromosome 13, 18, 21, X and Y based on multiplex ligation-dependent probe amplification (MLPA)". Eur. J. Hum. Genet. 13 (2): 171–175. doi:10.1038/sj.ejhg.5201307. PMID 15483643.
- Hochstenbach R, Meijer J, van de Brug J, Vossebeld-Hoff I, Jansen R, van der Luijt RB, Sinke RJ, Page-Christiaens GC, Ploos van Amstel JK, de Pater JM (2005). "Rapid detection of chromosomal aneuploidies in uncultured amniocytes by multiplex ligation-dependent probe amplification (MLPA)". Prenat. Diagn. 25 (11): 1032–1039. doi:10.1002/pd.1247. PMID 16231311.
- Illanes S, Avent N, Soothill PW (2005). "Cell-free fetal DNA in maternal plasma: an important advance to link fetal genetics to obstetric ultrasound". Ultrasound Obstet. Gynecol. 25 (4): 317–322. doi:10.1002/uog.1881. PMID 15789415.
- Giner-Delgado, C., Villatoro, S., Lerga-Jaso, J., Gayà-Vidal, M., Oliva, M., Castellano, D., ... & Olalde, I. (2019). Evolutionary and functional impact of common polymorphic inversions in the human genome. Nature communications, 10(1), 1-14. https://doi.org/10.1038/s41467-019-12173-x