n-Butylbenzene
n-Butylbenzene is the organic compound with the formula C6H5C4H9. Of two isomers of butylbenzene, n-butylbenzene consists of a phenyl group attached to the 1 position of a butyl group. It is a slightly greasy, colorless liquid.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Butylbenzene | |
| Other names
1-Butylbenzene 1-Phenylbutane | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ECHA InfoCard | 100.002.918 |
| EC Number |
|
PubChem CID |
|
| UNII | |
| UN number | 2709 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H14 | |
| Molar mass | 134.222 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 0.8601 g/cm3 at 20 °C |
| Melting point | −87.9 °C (−126.2 °F; 185.2 K) |
| Boiling point | 183.3 °C (361.9 °F; 456.4 K) |
| 11.8 mg/L | |
| Solubility | alcohol, ether, benzene |
| Hazards | |
| GHS pictograms |   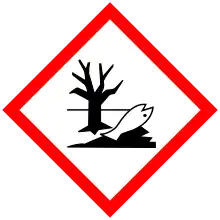 |
| GHS Signal word | Warning |
| H226, H315, H319, H400, H410 | |
| P210, P233, P240, P241, P242, P243, P264, P273, P280, P302+352, P303+361+353, P305+351+338, P321, P332+313, P337+313, P362, P370+378, P391, P403+235, P501 | |
| Flash point | 71 °C; 160 °F; 344 K |
| 410 °C (770 °F; 683 K) | |
| Related compounds | |
Related compounds |
iso-Butylbenzene, sec-Butylbenzene, tert-Butylbenzene |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The synthesis of n-butylbenzene by the reaction of chlorobenzene and butylmagnesium bromide was one of the first demonstrations of the Kumada coupling using nickel diphosphine catalysts.[1] This mild and efficient process contrasted with older methods.[2]
References
- Tamao, Kohei; Sumitani, Koji; Kumada, Makoto (1972). "Selective carbon-carbon bond formation by cross-coupling of Grignard reagents with organic halides. Catalysis by nickel-phosphine Complexes". Journal of the American Chemical Society. 94: 4374–6. doi:10.1021/ja00767a075.CS1 maint: uses authors parameter (link)
- R. R. Read, L. S. Foster, Alfred Russell, V. L. Simril (1945). "n-Butylbenzene". Org. Synth. 25: 11. doi:10.15227/orgsyn.025.0011.CS1 maint: uses authors parameter (link)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.