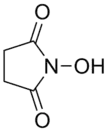
N-Hydroxysuccinimide
N-Hydroxysuccinimide (NHS) is an organic compound with the formula (CH2CO)2NOH. It is a white solid that is used as a reagent for preparing active esters in peptide synthesis. It can be synthesized by heating succinic anhydride with hydroxylamine or hydroxylamine hydrochloride.[1]
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
1-Hydroxy-2,5-pyrrolidinedione | |||
| Other names
1-hydroxypyrrolidine-2,5-dione, HOSu | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.025.456 | ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C4H5NO3 | |||
| Molar mass | 115.09 g/mol | ||
| Appearance | Colourless solid | ||
| Melting point | 95 °C (203 °F; 368 K) | ||
| Related compounds | |||
Related imides |
Succinimide N-Bromosuccinimide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Activating reagent
NHS is commonly found in organic chemistry or biochemistry where it is used as an activating reagent for carboxylic acids.[2] Activated acids (carboxylates) can react with amines to form amides for example, whereas a normal carboxylic acid would just form a salt with an amine.
Use
A common way to synthesize an NHS-activated acid is to mix NHS with the desired carboxylic acid and a small amount of an organic base in an anhydrous solvent. A coupling reagent such as dicyclohexylcarbodiimide (DCC) or ethyl(dimethylaminopropyl) carbodiimide (EDC) is then added to form a highly reactive activated acid intermediate. NHS reacts to form a less labile activated acid. The group itself is usually written as SuO- or -OSu in chemical notation. Such an ester with an acid and NHS, sometimes called succinate ester, is stable enough to be purified and stored at low temperatures in the absence of water and, as such, is commercially available. NHS esters are commonly used for protein modification (e.g. an NHS ester of fluorescein is commercially available, and can be added to a protein to obtain a fluorescently labeled protein in one simple reaction and purification step).
Alternatives
Some alternatives to NHS are the water-soluble analog sulfo-NHS, hydroxybenzotriazole (HOBt), 1-hydroxy-7-azabenzotriazole (HOAt), and pentafluorophenol.
References
- Knight, David W. (2001). "N-Hydroxysuccinimide". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rh069.
- Anderson, G.W.; Zimmerman, J. F.; Callahan, F. M. (1963). "N-Hydroxysuccinimide Esters in Peptide Synthesis". J. Am. Chem. Soc. 85 (19): 3039. doi:10.1021/ja00902a047.