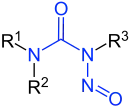
N-Nitrosamides
Nitrosamides are chemical compounds that contain of the chemical structure R1C(=X)N(–R2)–N=O, that is, a nitroso group bonded to the nitrogen of an amide or similar functional group.[1] Specific classes include the N-nitrosamides, N-nitrosoureas, N-nitrosoguanidines, and N-nitrosocarbamates. Nitrosamides are usually chemically reactive, metabolically unstable,[1] and often carcinogenic; however, in contrast to the N-nitrosamines, N-nitrosamides are not generally contaminants found in food.[1]
| Basic structure with blue emphasized functional group |
N-Nitrosamide derivates with blue emphasized functional group |
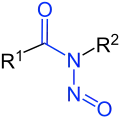 N-Nitrosamides |
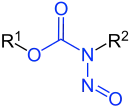 N-Nitrosocarbamates
|
| R1-R3 are hydrogen atoms or organic residues | |
Use
Various chloroethylnitrosoureas (such as N, N'-bis (2-chloroethyl)nitrosourea, BCNU) have obtained a medical use in the field of malignant tumors.[2] It is hypothesized that the efficacy against cancer cells is based on the alkylability of guanine cytosine centers in the sequences of the genetic material, especially the oncogenes.[2]
Synthesis
N-Nitrosamides can be prepared starting from N-monosubstituted carboxamides and the nitrosyl cation (which results from the nitrous cation in the presence of strong acids from the nitrous acid), here exemplified for N-methylacetamide (1).[3][4] The carboxamide reacts in a nucleophilic attack at the nitrosyl cation. After the elimination of a proton, an N-nitrosamide (2) is formed from the resulting cation:

Toxicity
The genotoxic effect of the N-nitroso compounds can be attributed to the formation of reactive electrophilic species in the metabolism.[5] The spontaneous decomposition of N-nitroso-ureas in the aqueous medium of the metabolism, here for example of 1-methylnitrosourea (3), produces diazonium or carbenium ions, respectively.[5] The decomposition occurs into isocyanic acid and methyldiazohydroxide. The rearrangement to the diazonium ion and the subsequent elimination of nitrogen results in a carbenium ion (4), which can alkylate nucleophilic intersections of the DNA.[5]
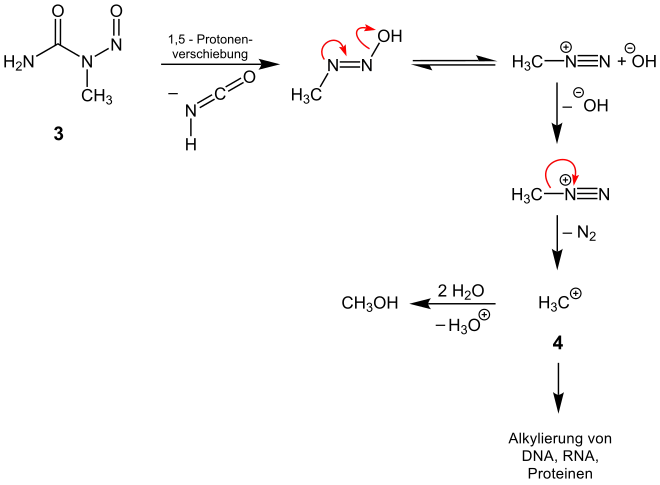
In the organism, the decomposition of N-nitroso ureas with a higher degree of substitution can proceed. An alternative possible formation of diazonium and carbenium ions is through the enzymatic reaction of nitrosamines.[5]
Typical accompanying symptoms during the medical cancer treatment via N-nitroso ureas are the impairment of bone marrow (damage of the stem cell compartment), lymphatic tissue and the gastrointestinal tract.[5]
References
- Hans Marquardt, Siegfried G. Schäfer (Hrsg.): Lehrbuch der Toxikologie. 2. Auflage, Wissenschaftliche Verlagsgesellschaft mbH Stuttgart, 2004, ISBN 3-8047-1777-2, S. 747.
- Hans Marquardt, Siegfried G. Schäfer (Hrsg.): Lehrbuch der Toxikologie. 2. Auflage, Wissenschaftliche Verlagsgesellschaft mbH Stuttgart, 2004, ISBN 3-8047-1777-2, S. 752–753.
- Adalbert Wollrab: Organische Chemie. Eine Einführung für Lehramts- und Nebenfachstudenten. 4. Auflage, Springer-Verlag Berlin Heidelberg 2014, ISBN 978-3-642-45144-7, S. 898.
- Heinz G. O. Becker, Rainer Beckert, Werner Berger, Günter Domschke, Egon Fanghänel, Mechthild Fischer, Frithjof Gentz, Karl Gewald, Reiner Gluch, Wolf D. Habicher, Hans-Joachim Knölker, Roland Mayer, Peter Meth, Klaus Müller, Dietrich Pavel, Hermann Schmidt, Karl Schollberg, Klaus Schwetlick, Erika Seiler, Günter Zeppenfeld: Organikum. 24. Auflage, Wiley-VCH Verlag GmbH & Co.KGaA, Weinheim 2015, ISBN 978-3-527-33968-6, S. 648.
- Hans Marquardt, Siegfried G. Schäfer (Hrsg.): Lehrbuch der Toxikologie. 2. Auflage, Wissenschaftliche Verlagsgesellschaft mbH Stuttgart, 2004, ISBN 3-8047-1777-2, S. 753–758.
External links
- "nitrosamide". Wiktionary. Retrieved 2017-08-28.