Guanidine
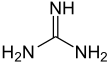
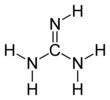
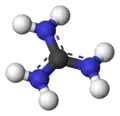
Guanidine is the compound with the formula HNC(NH2)2. It is a colourless solid that dissolves in polar solvents. It is a strong base that is used in the production of plastics and explosives. It is found in urine as a normal product of protein metabolism. A guanidine moiety also appears in larger organic molecules, including on the side chain of arginine.
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
Guanidine[1] Iminomethanediamine | |||
| Identifiers | |||
3D model (JSmol) |
|||
| 506044 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.003.656 | ||
| EC Number |
| ||
| 100679 | |||
| MeSH | Guanidine | ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| CH5N3 | |||
| Molar mass | 59.072 g·mol−1 | ||
| Melting point | 50 °C (122 °F; 323 K) | ||
| log P | −1.251 | ||
| Acidity (pKa) | 13.6 | ||
| Conjugate acid | Guanidinium | ||
| Thermochemistry | |||
Std enthalpy of formation (ΔfH⦵298) |
−57 – −55 kJ mol−1 | ||
Std enthalpy of combustion (ΔcH⦵298) |
−1.0511 – −1.0531 MJ mol−1 | ||
| Pharmacology | |||
| Pharmacokinetics: | |||
| 7–8 hours | |||
| Hazards | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
475 mg/kg (oral, rat)[2] | ||
| Related compounds | |||
Related compounds |
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Structure
Guanidine can be thought of as a nitrogenous analogue of carbonic acid. That is, the C=O group in carbonic acid is replaced by a C=NH group, and each OH is replaced by a NH
2 group.[3] Isobutylene can be seen as the carbon analogue in much the same way. A detailed crystallographic analysis of guanidine was elucidated 148 years after its first synthesis, despite the simplicity of the molecule.[4] In 2013, the positions of the hydrogen atoms and their displacement parameters were accurately determined using single-crystal neutron diffraction.[5]
Production
Guanidine can be obtained from natural sources, being first isolated by Adolph Strecker via the degradation of guanine.[6] It was first synthesized in 1861 by the oxidative degradation of an aromatic natural product, guanine, isolated from Peruvian guano.[7]
A laboratory method of producing guanidine is gentle (180-190 °C) thermal decomposition of dry ammonium thiocyanate in anhydrous conditions:
The commercial route involves a two step process starting with the reaction of dicyandiamide with ammonium salts. Via the intermediacy of biguanidine, this ammonolysis step affords salts of the guanidinium cation (see below). In the second step, the salt is treated with base, such as sodium methoxide.[6]
Chemistry
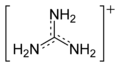
Guanidinium cation
With a pKb of 0.4, guanidine is a strong base. Most guanidine derivatives are in fact salts containing the conjugate acid.
The conjugate acid is called the guanidinium cation, (C(NH
2)+
3). This planar, symmetric ion consists of three amino groups each bonded to the central carbon atom with a covalent bond of order 4/3. It is a highly stable +1 cation in aqueous solution due to the efficient resonance stabilization of the charge and efficient solvation by water molecules. As a result, its pKa is 13.6[8] meaning that guanidine is a very strong base in water; in neutral water, it exists almost exclusively as guanidinium.
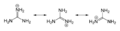
canonical forms
Testing for guanidine
Guanidine can be selectively detected using sodium 1,2-naphthoquinone-4-sulfonic acid (Folin's reagent) and acidified urea.[9]
Uses
Industry
The main salt of commercial interest is the nitrate [C(NH
2)3]NO
3. It is used as a propellant, for example in air bags.
Biochemistry
Guanidine exists protonated, as guanidinium, in solution at physiological pH.
Guanidinium chloride (also known as guanidine hydrochloride) has chaotropic properties and is used to denature proteins. Guanidinium chloride is known to denature proteins with a linear relationship between concentration and free energy of unfolding. In aqueous solutions containing 6 M guanidinium chloride, almost all proteins lose their entire secondary structure and become randomly coiled peptide chains. Guanidinium thiocyanate is also used for its denaturing effect on various biological samples.
Guanidinium chloride[10] is used as an adjuvant in treatment of botulism, introduced in 1968,[11] but now its role is considered controversial[12] – because in some patients there was no improvement after the administration of this drug.
Other
Guanidinium hydroxide is the active ingredient in some non-lye hair relaxers.
Guanidine derivatives
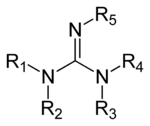
Guanidines are a group of organic compounds sharing a common functional group with the general structure (R
1R
2N)(R
3R
4N)C=N−R
5. The central bond within this group is that of an imine, and the group is related structurally to amidines and ureas. Examples of guanidines are arginine, triazabicyclodecene, saxitoxin, and creatine.
See also
- Category:Guanidines
- Sakaguchi test
- Y-aromaticity
- Amidine
References
- "Guanidine – Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 16 September 2004. Identification. Retrieved 29 February 2012.
- "Guanidine hydrochloride". ChemIDplus. National Library of Medicine.
- Goebel, M.; Klapoetke, T. M. (2007). "First structural characterization of guanidine". Chem. Commun. 43 (30): 3180–2. doi:10.1039/B705100J. PMID 17653381.
- Yamada, T.; Liu, X.; Englert, U.; Yamane, H.; Dronskowski, R. (2009). "Solid-state structure of free base guanidine achieved at last". Chem. Eur. J. 15 (23): 5651–5. doi:10.1002/chem.200900508. PMID 19388036.
- Sawinski, P. K.; Meven, M.; Englert, U.; Dronskowski, R. (2013). "Single-Crystal Neutron Diffraction Study on Guanidine, CN3H5". Cryst. Growth Des. 13 (4): 1730–5. doi:10.1021/cg400054k.
- Güthner, Thomas; Mertschenk, Bernd; Schulz, Bernd. "Guanidine and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a12_545.pub2.
- Strecker, A. (1861). "Untersuchungen über die chemischen Beziehungen zwischen Guanin, Xanthin, Theobromin, Caffeïn und Kreatinin" [Studies on the chemical relationships between guanine, xanthine, theobromine, caffeine and creatinine]. Liebigs Ann. Chem. 118 (2): 151–177. doi:10.1002/jlac.18611180203.
- Perrin, D. D. (1972). Dissociation Constants of Organic Bases in Aqueous Solution (Supplement ed.). London: Butterworths..
- Sullivan, M. X. (1935-10-01). "A Colorimetric Test for Guanidine". Proceedings of the Society for Experimental Biology and Medicine. 33 (1): 106–108. doi:10.3181/00379727-33-8270C. ISSN 0037-9727. S2CID 88290359.
- Kaplan, J. E.; Davis, L. E.; Narayan, V.; Koster, J.; Katzenstein, D. (1979). "Botulism, type A, and treatment with guanidine". Annals of Neurology. 6 (1): 69–71. doi:10.1002/ana.410060117. PMID 389150. S2CID 42901888.
- Puggiari, Marcello; Cherington, Michael (1978). "Botulism and Guanidine: Ten Years Later". J. Am. Med. Assoc. 240 (21): 2276–7. doi:10.1001/jama.1978.03290210058027. PMID 702753.
- Brook, Itzhak (2001). Pediatric Anaerobic Infections: Diagnosis and Management (3rd ed.). Taylor & Francis. p. 529. ISBN 0824741862.
- Witters, L. A. (2001). "The blooming of the French lilac". Journal of Clinical Investigation. 108 (8): 1105–7. doi:10.1172/JCI14178. PMC 209536. PMID 11602616.